Speak directly to the analyst to clarify any post sales queries you may have.
Rocuronium bromide API is becoming a strategic essential as reliability, compliance, and continuity define value beyond commodity pricing
Rocuronium bromide API sits at the center of a critical clinical workflow: reliable neuromuscular blockade to enable safe and efficient airway management and surgical procedures. Because the finished dosage forms must perform consistently under urgent conditions, the API behind them is held to exceptionally high expectations for identity, purity, and batch-to-batch reproducibility. This makes the rocuronium bromide API market less about discretionary demand and more about trust, continuity, and uncompromising manufacturing discipline.In recent years, stakeholders across anesthesia, critical care, and hospital pharmacy have paid heightened attention to supply resilience for essential injectables. As a result, rocuronium bromide API buyers are scrutinizing not only technical specifications but also the strength of quality systems, change-control rigor, impurity management strategies, and the ability to support regulatory filings across multiple jurisdictions.
Against this backdrop, the competitive conversation has expanded. Procurement teams and finished-dose manufacturers increasingly weigh multi-site qualification, traceability of key starting materials, and responsiveness during audits alongside price and lead time. Consequently, the market’s executive lens is shifting toward operational credibility and risk-adjusted value, particularly as geopolitical and trade dynamics introduce fresh complexity into cross-border sourcing.
Quality-first procurement, dual-sourcing discipline, and stricter regulatory expectations are reshaping how rocuronium bromide API suppliers compete
The rocuronium bromide API landscape is undergoing transformative shifts driven by a convergence of quality expectations, supply-chain reconfiguration, and regulatory maturity. One of the most consequential changes is the intensified focus on end-to-end control of impurities and degradants, especially for sterile injectable applications where downstream formulation offers limited ability to compensate for upstream variability. This has elevated the importance of robust analytical methods, validated cleaning processes, and disciplined deviation management.At the same time, buyers are changing how they qualify suppliers. Rather than treating API sourcing as a single-vendor relationship optimized for cost, many manufacturers are moving toward dual sourcing strategies that include contingency planning, approved alternate manufacturing lines, and improved visibility into upstream raw materials. This shift is partly a response to recurring disruptions in global logistics and partly a recognition that regulatory remediation can be costly and time-consuming when a sole supplier encounters quality issues.
Regulatory expectations are also evolving in ways that influence competitive positioning. Authorities are emphasizing data integrity, lifecycle management, and proactive change notification, which can affect how quickly manufacturers implement process improvements or scale production. As these expectations rise, suppliers with mature quality cultures and strong documentation practices gain an advantage, particularly when supporting customers that need rapid dossier updates or multi-country variations.
Finally, sustainability and responsible manufacturing are becoming more prominent in procurement and partnership discussions. While rocuronium bromide API is primarily evaluated through a clinical reliability lens, environmental controls, solvent recovery, waste management, and ethical sourcing are increasingly visible in supplier evaluations. Collectively, these shifts are redefining what “best-in-class” means, pushing the market toward fewer surprises, more transparency, and higher operational resilience.
U.S. tariff dynamics in 2025 are changing landed-cost math, contract behavior, and supply-chain regionalization for rocuronium bromide API
The cumulative impact of United States tariffs in 2025 is expected to influence rocuronium bromide API sourcing decisions through both direct and indirect channels. Even when tariff classifications do not neatly map onto a specific API line item, the broader trade environment can affect landed cost through duties on intermediates, packaging components, chemical precursors, and select services embedded in cross-border production. This creates a situation where the true cost impact is often realized across a basket of inputs rather than a single invoice line.As tariff pressure accumulates, procurement organizations are likely to intensify total-cost modeling and revisit supplier mix decisions. Contract structures may evolve toward shorter pricing windows, index-linked adjustments, or negotiated corridors that share risk between API suppliers and finished-dose manufacturers. In parallel, inventory strategies may shift, with some buyers increasing safety stock for high-criticality products to buffer against customs delays, policy shifts, or sudden cost spikes.
Tariffs can also accelerate supply-chain regionalization. Finished-dose manufacturers serving the U.S. market may prioritize API sources with more predictable trade exposure, stronger documentation for customs clearance, and proven responsiveness in the event of reclassification or compliance queries. Additionally, tariff-related uncertainty can heighten the strategic value of secondary suppliers already qualified under U.S. regulatory expectations, because switching costs are magnified when lead times and regulatory updates are involved.
Over time, the most significant effect may be behavioral: tariffs encourage procurement and quality teams to collaborate earlier and more tightly. When cost volatility rises, companies tend to reduce surprises by integrating trade compliance, quality assurance, and sourcing strategy into a single governance rhythm. For rocuronium bromide API, this integrated approach becomes a practical necessity, helping organizations maintain clinical continuity while managing financial exposure in a policy environment that can change faster than validation cycles.
Segmentation patterns show rocuronium bromide API demand is governed by grade expectations, injectable performance needs, and buyer risk tolerance
Segmentation insights for rocuronium bromide API reveal a market shaped by how buyers translate clinical-criticality into sourcing rules. When viewed by product grade and compendial alignment, demand increasingly favors suppliers that can demonstrate consistent conformance to pharmacopoeial requirements and provide complete analytical packages that support rapid quality review. Buyers are placing greater weight on impurity profiling, residual solvent control, and stability signaling because these elements simplify downstream qualification and reduce the risk of post-approval changes.When considered through the lens of application and end use, purchasing behavior is strongly tied to hospital and surgical utilization patterns, as well as the preparedness expectations embedded in anesthesia workflows. This creates a preference for uninterrupted supply and conservative change management, especially where any interruption can cascade into substitution protocols and operational strain. Consequently, application-driven segmentation tends to reward suppliers that can maintain dependable batch cadence and demonstrate robust business continuity planning.
From a formulation and route-of-administration perspective, the injectable nature of rocuronium bromide magnifies the importance of API attributes that affect solubility, particulate control, and compatibility with sterile processing. Even though sterile manufacture occurs at the finished-dose stage, API suppliers that provide stronger contamination control narratives and tighter variability bands often reduce investigation burden for their customers. This practical advantage shows up in supplier selection and requalification decisions.
Segmentation by customer type and purchasing model further clarifies competitive dynamics. Large pharmaceutical manufacturers and established generic players typically prefer suppliers that can support audits at scale, manage technical queries quickly, and deliver documentation suited for multiple regulatory dossiers. Meanwhile, contract manufacturers and smaller finished-dose developers often value responsive technical support, flexible minimum order quantities, and predictable lead times. Across these segments, the differentiators converge on reliability, documentation quality, and the ability to absorb change without destabilizing supply.
Regional buying behavior diverges across the Americas, EMEA, and Asia-Pacific as compliance posture and supply resilience shape qualification standards
Regional insights indicate that rocuronium bromide API demand and sourcing criteria vary materially based on regulatory cadence, procurement norms, and manufacturing concentration. In the Americas, buyer priorities often center on regulatory readiness, audit responsiveness, and continuity planning, reflecting strong institutional expectations for supply assurance in hospital channels. This environment tends to favor suppliers that can support rigorous quality agreements and demonstrate disciplined change control.Across Europe, the Middle East, and Africa, procurement is shaped by a combination of centralized purchasing structures, mature regulatory frameworks, and heightened sensitivity to documentation completeness. Buyers often emphasize transparency in the supply chain, robust pharmacovigilance alignment, and reliable technical support for dossier maintenance. In parts of the region where tendering influences purchasing behavior, suppliers that can balance competitive pricing with dependable delivery and stable specifications are better positioned.
In Asia-Pacific, manufacturing depth and export orientation create a distinct set of dynamics. The region includes major API production hubs as well as rapidly evolving domestic pharmaceutical markets, which together drive both supply availability and competitive intensity. Buyers increasingly evaluate suppliers on global compliance track records, readiness for multi-country inspections, and the resilience of upstream sourcing for intermediates. Additionally, as healthcare access expands and surgical volumes rise in several markets, the focus on uninterrupted availability becomes more prominent, reinforcing the value of scalable production and predictable logistics.
Taken together, regional differences highlight a common trajectory: purchasers everywhere are tightening qualification standards while seeking more resilient supply configurations. The most successful suppliers adapt their commercial and quality engagement to regional expectations, offering not only compliant material but also the documentation, responsiveness, and operational predictability that regulators and healthcare systems increasingly demand.
Leading rocuronium bromide API suppliers stand out through quality maturity, regulatory support depth, and resilient manufacturing footprints
Key company insights in rocuronium bromide API point to competition that is increasingly defined by quality maturity, documentation strength, and the ability to operate reliably under scrutiny. Companies that consistently win long-term relationships typically demonstrate robust process validation, clear impurity control strategies, and a track record of responsive deviation handling. In a market linked to essential clinical care, buyers interpret these capabilities as indicators of low operational risk.Another differentiator is how effectively companies support customer regulatory needs. Suppliers that provide well-structured drug master file support where applicable, timely responses to customer questionnaires, and disciplined change notifications reduce friction for finished-dose manufacturers maintaining approvals across multiple markets. This capability becomes particularly valuable when customers face tight timelines for variations, site transfers, or post-approval commitments.
Manufacturing footprint and supply-chain design also influence perceived reliability. Companies with redundant capacity, qualified alternate lines, and stronger control over key starting materials tend to be viewed as more resilient partners, especially when global logistics become volatile. Conversely, suppliers with limited transparency into upstream sourcing may face greater scrutiny, even if their pricing is attractive.
Finally, commercial behavior matters in a product category where continuity can outweigh short-term savings. Companies that invest in proactive communication, realistic lead-time commitments, and collaborative quality agreements often build durable trust. In practice, the strongest players treat the relationship as a shared responsibility for patient-critical availability, aligning technical rigor with operational predictability.
Industry leaders can reduce risk by strengthening dual sourcing, tariff-aware contracts, deeper quality transparency, and cross-functional governance
Industry leaders can strengthen their position by operationalizing a resilience-first sourcing strategy. That begins with qualifying at least one alternate source where feasible and ensuring the alternate is not merely approved on paper but is supported by periodic technical engagement, audit cadence, and realistic allocation planning. When product criticality is high, an alternate that cannot scale or cannot maintain documentation quality offers limited protection.Next, organizations should upgrade their total-cost governance to account for tariff volatility, customs friction, and upstream input exposure. This means integrating trade compliance into supplier selection and embedding adjustment mechanisms into contracts so that disputes do not arise during periods of rapid policy change. In parallel, aligning safety-stock policy with clinical criticality and supplier performance helps avoid emergency procurement that typically increases both cost and quality risk.
Quality and regulatory teams should also push for deeper technical transparency. Requesting tighter impurity trend reporting, process capability narratives, and clear change-control commitments can reduce surprises. Where supplier capabilities are strong, collaborative continuous improvement programs can stabilize yields and reduce variability, which ultimately supports better on-time delivery and fewer batch investigations.
Finally, leaders should invest in cross-functional readiness. Establishing a shared operating rhythm across procurement, quality, regulatory, and manufacturing enables faster responses when deviations, logistics disruptions, or tariff changes occur. In essential injectable supply chains, speed and alignment are competitive advantages, and the organizations that institutionalize them are better equipped to protect patients while sustaining business performance.
A triangulated methodology blends primary stakeholder input with regulatory, technical, and trade documentation to ensure decision-ready insights
The research methodology for this analysis combines structured primary engagement with rigorous secondary validation to ensure relevance to both technical and executive audiences. Primary inputs include interviews and discussions with stakeholders across API manufacturing, quality assurance, regulatory affairs, procurement, and finished-dose formulation, focusing on decision criteria, qualification hurdles, and operational pain points.Secondary research incorporates a broad review of regulatory publications, pharmacopoeial expectations, public inspection and compliance communications where available, corporate disclosures, scientific and technical literature on synthesis and impurity control, and trade policy documentation relevant to cross-border chemical sourcing. These inputs help contextualize how requirements and operating conditions are evolving.
The analysis uses triangulation to reconcile differing viewpoints and to separate structural trends from short-term noise. Company positioning is assessed through observable indicators such as manufacturing and quality-system signals, documentation readiness, and supply continuity practices, rather than relying on single-source claims. Throughout, findings are organized to reflect how buyers make decisions in practice, linking technical attributes to procurement outcomes.
Finally, editorial quality control is applied to ensure internal consistency, clarity, and decision utility. Emphasis is placed on actionable interpretation, enabling readers to translate market observations into supplier strategies, qualification priorities, and risk management actions aligned with rocuronium bromide API’s essential role in care delivery.
Rocuronium bromide API success now depends on resilient sourcing, stronger quality systems, and tariff-aware decision-making discipline
Rocuronium bromide API remains a clinically essential input where performance expectations and continuity needs create a high bar for suppliers and a careful decision framework for buyers. The market is moving away from purely price-led sourcing toward a more disciplined evaluation of quality maturity, documentation readiness, and operational resilience.Transformative shifts in procurement practices, coupled with heightened regulatory expectations, are raising the value of transparent partnerships and robust change control. At the same time, the cumulative effects of U.S. tariff dynamics in 2025 are pushing organizations to refine total-cost models, redesign contracts, and reconsider the geographic shape of their supply chains.
Ultimately, success in this environment depends on aligning technical rigor with supply reliability. Companies that invest in resilient sourcing architectures, proactive regulatory support, and cross-functional governance will be better positioned to sustain dependable supply while navigating policy and logistics complexity.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Rocuronium Bromide API Market
Companies Mentioned
The key companies profiled in this Rocuronium Bromide API market report include:- Aurobindo Pharma
- Changzhou Comwin Fine Chemicals Co., Ltd
- Curia (formerly AMRI)
- Dr. Reddy's Laboratories Ltd.
- Euticals
- Fresenius Kabi AG
- Global Calcium
- Hikma Pharmaceuticals PLC
- Jiangsu Run'an Pharmaceutical Co., Ltd.
- Jiangxi Bioman Pharma Limited
- Jubilant Life Sciences Ltd.
- Lupin Ltd.
- Pfizer Inc.
- Reliance Life Sciences Private Limited
- Sandoz International GmbH
- Shaoxing Hantai Pharma
- Synnat Pharma Private Limited
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- Zhejiang Xianju
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
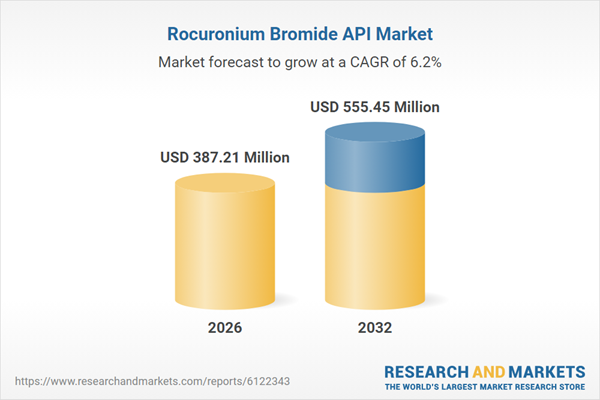
| Estimated Market Value ( USD | $ 387.21 Million |
| Forecasted Market Value ( USD | $ 555.45 Million |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |