Speak directly to the analyst to clarify any post sales queries you may have.
Clinical molecular imaging systems are evolving from diagnostic tools into precision-medicine platforms that influence therapy decisions, workflows, and outcomes
Clinical molecular imaging systems have become central to how health systems and research organizations detect disease earlier, characterize biology noninvasively, and tailor therapy to patient-specific targets. Rather than simply producing anatomical pictures, modern platforms translate tracer kinetics, receptor density, metabolism, perfusion, and cellular processes into measurable signals that can guide diagnosis, staging, response assessment, and surveillance. As precision oncology expands and neurology and cardiology adopt quantitative imaging more broadly, molecular imaging is increasingly viewed as an enabling infrastructure for precision medicine rather than an optional adjunct.The category is also being reshaped by operational realities. Sites are asked to do more with the same staff, deliver consistent quality across distributed networks, and meet stricter expectations for dose management and safety while keeping throughput high. In parallel, the shift toward quantitative imaging, standardized protocols, and harmonized reporting is driving demand for systems that can support robust calibration, repeatable reconstruction, and analytics-ready outputs. Consequently, buyers are not only comparing scanner specifications; they are evaluating ecosystems-software, service, radiopharmacy compatibility, cybersecurity, data pathways, and vendor roadmaps.
Against this backdrop, the clinical molecular imaging system landscape is at an inflection point. Radiotheranostics growth is linking diagnostics and therapy workflows more tightly than ever, and advances in detectors, reconstruction, and AI are changing what “state-of-the-art” means in daily practice. The executive summary that follows synthesizes the most consequential shifts, policy pressures, segmentation dynamics, regional differences, competitive themes, and practical actions that industry leaders can take to strengthen clinical impact and business resilience.
Quantification, radiotheranostics convergence, detector innovation, and data governance are redefining what leadership expects from molecular imaging platforms
The landscape is undergoing transformative shifts that extend beyond incremental hardware upgrades. First, the move from qualitative interpretation to quantitative decision support is accelerating. Clinicians increasingly expect consistent standardized uptake values, kinetic modeling options, and longitudinal comparability, which elevates the importance of calibration routines, harmonized reconstruction, and cross-system reproducibility. This shift is reinforced by multi-center trials and health networks that need comparable metrics across sites, pushing vendors to embed quality controls, phantom-based validation, and automation that reduces operator variability.Second, radiotheranostics is reshaping system requirements and departmental collaboration. Diagnostic imaging is now frequently paired with therapy planning, dosimetry, and treatment monitoring. This convergence increases demand for platforms that integrate dosimetry workflows, support theranostic tracer families, and interface smoothly with radiopharmacy production, scheduling, and safety processes. As a result, the buying center expands beyond radiology and nuclear medicine to include oncology service lines, pharmacy leadership, radiation safety, and finance teams focused on end-to-end patient pathways.
Third, detector innovation and reconstruction advances are changing the throughput-versus-quality trade-off. Digital detection, time-of-flight improvements, and motion correction have reduced acquisition time or improved image quality at similar doses, enabling protocol redesign and potentially better patient experience. At the same time, AI-enabled reconstruction and post-processing are being positioned as upgrades that can extend the useful life of installed bases, though customers are scrutinizing validation, bias risks, and performance across diverse patient populations.
Finally, connectivity and data governance have become decisive. Molecular imaging outputs are increasingly used in enterprise analytics, research repositories, and AI development pipelines. This heightens expectations for secure interoperability, DICOM consistency, structured reporting, and integration with PACS, VNA, RIS, and clinical trial management systems. Cybersecurity posture and software lifecycle support now influence vendor selection nearly as strongly as technical performance, especially for health systems managing large fleets and strict compliance requirements.
US tariff uncertainty in 2025 is poised to affect system pricing, lead times, and service predictability, elevating supply-chain transparency as a buying criterion
United States tariff dynamics anticipated for 2025 introduce a layer of procurement uncertainty for clinical molecular imaging systems, particularly where complex supply chains span multiple regions for detectors, semiconductors, precision electronics, shielding components, and specialized subassemblies. Even when final assembly occurs domestically, upstream parts may be tariff-exposed, which can translate into price volatility, longer lead times, or shifting configuration options. For buyers, the practical consequence is that capital planning needs tighter coordination with vendor sourcing strategies and clearer language in quotes regarding validity periods, escalation clauses, and substitution policies.Tariff-related pressure can also reshape service economics. If replacement parts or consumables face higher import costs, service contracts may adjust over renewal cycles, and on-demand parts pricing can become less predictable. Providers may respond by seeking more comprehensive coverage, prioritizing uptime guarantees, and negotiating parts availability commitments. In parallel, vendors may increase regional warehousing, qualify alternate suppliers, or redesign components to reduce exposure. These adaptations can improve resilience over time, but during transition phases they may temporarily affect installation schedules and field service responsiveness.
From an industry standpoint, tariffs can accelerate localization efforts and deepen partnerships with domestic manufacturing and logistics networks. However, localization is not immediate for highly specialized detector technologies and regulated components, so near-term strategy often emphasizes inventory planning, dual-sourcing, and proactive forecasting. For health systems, a prudent approach in 2025 is to align purchasing timelines with tariff decision points, request transparency on country-of-origin risk by subsystem, and build contingency plans for phased deployments. In effect, tariffs become not just a pricing factor but a strategic variable influencing vendor selection, lifecycle cost predictability, and continuity of clinical operations.
Segmentation reveals that modality, end-user priorities, applications, and procurement models now converge on throughput, quantification rigor, and workflow integration
Segmentation dynamics in clinical molecular imaging systems are increasingly defined by how technology choices align to clinical programs, operational constraints, and data ambitions. By modality, PET systems continue to be prioritized where oncology pathways, radiotheranostics expansion, and quantitative response monitoring drive value, while SPECT remains essential for broad nuclear medicine access, cardiology workflows, and cost-sensitive environments seeking high utilization with proven tracer availability. Hybrid configurations such as PET/CT and SPECT/CT are frequently evaluated as default choices for clinical breadth, whereas PET/MR tends to be adopted in centers with strong neurology, pediatric, or research mandates where soft-tissue contrast and reduced radiation are strategic differentiators.By detector technology and performance tier, decision-making increasingly focuses on consistency and throughput rather than peak specifications alone. Digital and time-of-flight capable PET platforms are considered where faster protocols, lower dose strategies, or high patient volumes are operational priorities. Conversely, sites with constrained budgets may favor established analog designs while investing in software upgrades that improve reconstruction and quantification. In SPECT, camera design and collimation options still matter, but buyers increasingly assess workflow automation, attenuation correction via CT integration, and reliability in high-throughput cardiac programs.
By end user, academic medical centers and research institutes typically emphasize advanced quantification, multi-tracer flexibility, and trial readiness, including harmonization tools that support standardized imaging biomarkers. Hospitals and integrated delivery networks often prioritize operational standardization across sites, service coverage, and predictable lifecycle costs, especially when deploying systems across multiple campuses. Diagnostic imaging centers and outpatient providers tend to focus on throughput, scheduling efficiency, and payor-aligned protocol sets, while specialty oncology centers increasingly evaluate molecular imaging as a backbone for theranostics clinics, requiring tight integration of imaging, dosimetry, and therapy scheduling.
By application area, oncology remains the anchor for PET adoption due to staging and therapy monitoring needs, while neurology growth is propelled by the expanding role of molecular biomarkers in neurodegenerative disease evaluation and research translation. Cardiology continues to support strong demand for SPECT and selective PET growth where perfusion and viability workflows benefit from quantification and improved attenuation correction. Across applications, software segmentation-reconstruction, motion correction, AI-enabled analytics, and structured reporting-has become a decisive layer that can either amplify hardware performance or limit clinical scalability if underpowered.
By workflow environment and procurement model, the market is differentiating between greenfield installations that prioritize future-proof architectures and upgrades that seek to maximize existing room infrastructure. Leasing, managed equipment services, and subscription-like software licensing are increasingly evaluated to manage capital constraints and keep features current. Across these segments, the systems that win are those that reduce variability, accelerate patient throughput, and produce analytics-ready data without increasing staffing burden.
Regional realities across the Americas, EMEA, and Asia-Pacific shape adoption through reimbursement, tracer access, infrastructure maturity, and service readiness
Regional dynamics reflect uneven maturity in installed base, radiopharmaceutical access, reimbursement patterns, and infrastructure readiness. In the Americas, large integrated health systems emphasize fleet standardization, cybersecurity, service performance, and quantitative consistency across multi-site networks. The region’s strong momentum in theranostics and oncology care pathways reinforces demand for PET/CT upgrades and software capabilities that support dosimetry-adjacent workflows, while outpatient expansion keeps pressure on throughput and scheduling efficiency.Across Europe, the Middle East, and Africa, procurement is shaped by a mix of public health budgeting, cross-border regulatory considerations, and heterogeneous access to tracers and cyclotron infrastructure. Western European markets often prioritize harmonization for multi-center clinical studies and guideline-driven protocol consistency, which strengthens demand for standardized quantification and vendor support for compliance documentation. In parts of the Middle East, rapid healthcare infrastructure investment can accelerate adoption of advanced hybrid imaging, while several African markets face constraints that elevate the importance of durable systems, service accessibility, and training models that sustain performance with limited specialist availability.
In Asia-Pacific, growth in tertiary care capacity and expanding oncology and neurology programs are driving broad-based demand, but priorities vary sharply by country and care setting. Mature markets frequently emphasize advanced detector performance, digital workflows, and integration with enterprise imaging and AI initiatives. Developing markets may focus on scalable deployment, cost-effective configurations, and reliable uptime, with vendor training and local service infrastructure acting as decisive differentiators. Across the region, increasing participation in global clinical research further elevates the need for protocol standardization and data interoperability, making software ecosystems and compliance readiness as important as the scanner itself.
Company differentiation increasingly hinges on ecosystem depth - software, upgrades, service excellence, cybersecurity support, and theranostics-aligned partnerships
Competition among leading companies is increasingly defined by platform ecosystems rather than standalone scanners. Vendors are differentiating through detector roadmaps, reconstruction and quantification software, motion correction, and workflow automation that reduces scan-to-report time. A key theme is the effort to convert technical performance into operational outcomes-higher daily throughput, more consistent quantitative metrics, and easier protocol management across distributed networks. As customers demand multi-year continuity, vendors also compete on upgradeability, allowing sites to add software features, improve reconstruction, or extend analytics without replacing full hardware footprints.Service strategy has become a primary battleground. Providers are weighing response times, parts availability, remote diagnostics, and preventive maintenance programs that reduce downtime. Vendors with stronger regional service presence and mature remote monitoring can translate reliability into measurable clinical capacity. At the same time, cybersecurity support and software patch cadence have emerged as differentiators, particularly for enterprise customers operating large fleets and seeking consistent governance across modalities.
Partnerships across the molecular imaging value chain are also shaping competitive positioning. Relationships with radiopharmaceutical producers, dosimetry software providers, and clinical application specialists can expand a vendor’s relevance in theranostics pathways. Companies that invest in clinical education, protocol optimization, and evidence generation strengthen customer stickiness, while open integration strategies that support interoperability can win favor among research-driven institutions. Ultimately, the strongest positions are held by companies that align hardware innovation, software evolution, and service execution into a coherent lifecycle proposition that meets both clinical and operational stakeholders.
Leaders can win by aligning platforms to theranostics and quantification goals, tightening lifecycle contracts, and operationalizing interoperability and training
Industry leaders can strengthen position by treating molecular imaging as a longitudinal capability rather than a one-time capital purchase. Start by aligning modality choices and upgrade paths to clinical strategy, especially where theranostics, oncology center growth, or neurodegenerative disease initiatives demand robust quantification and protocol consistency. Standardizing key protocols across sites and building governance for calibration, reconstruction settings, and reporting reduces variability and improves comparability, which benefits both clinical decisions and research participation.Next, de-risk procurement under supply-chain uncertainty by requiring transparency on component sourcing, lead times, and substitution rules. Contracting should emphasize lifecycle predictability through clearly defined service-level commitments, parts availability language, cybersecurity patch obligations, and options for software feature expansion. When feasible, consider phased rollouts that protect clinical continuity, pairing early deployments with staff training and workflow redesign so throughput gains are realized in practice rather than remaining theoretical.
Operationally, prioritize automation and interoperability to reduce staffing strain. Implement workflow tools that streamline scheduling, dose management, motion correction, and structured reporting, and ensure outputs integrate cleanly with enterprise imaging and analytics systems. Finally, invest in talent and cross-department coordination by formalizing collaboration among nuclear medicine, radiology, oncology, radiopharmacy, and IT security teams. Organizations that build these connective processes will be better positioned to scale radiotheranostics programs, support clinical trials, and maintain consistent quality across expanding networks.
A triangulated methodology combining technical literature, policy review, and stakeholder interviews translates complex imaging innovation into decision-ready insight
The research methodology for this analysis integrates structured secondary research with expert-informed primary validation to develop a practical view of the clinical molecular imaging system environment. Secondary research draws on regulatory and policy documentation, peer-reviewed clinical and engineering literature, standards and guidance from professional bodies, public company disclosures, product documentation, and procurement-related materials to map technology evolution, workflow practices, and compliance considerations.Primary research inputs are developed through interviews and structured consultations with stakeholders across the value chain, including clinical leaders in nuclear medicine and radiology, medical physicists, radiopharmacy and radiation safety professionals, healthcare procurement teams, and executives from technology and service providers. These perspectives are used to validate adoption drivers, identify operational pain points, and clarify how purchasing decisions are made under real-world constraints such as staffing, uptime requirements, tracer logistics, and IT governance.
Findings are synthesized using triangulation to ensure consistency across sources and to reconcile differing viewpoints. Segmentation and regional frameworks are applied to organize insights by modality, end-user needs, application demands, and geographic operating conditions, while competitive analysis emphasizes lifecycle value, ecosystem completeness, and service execution. Throughout, the approach prioritizes decision relevance, highlighting actionable implications for strategy, procurement, and operational planning without relying on speculative assumptions.
Clinical molecular imaging success now depends on quantification discipline, lifecycle resilience, and enterprise integration as technology and policy pressures intensify
Clinical molecular imaging systems are becoming foundational to precision medicine as quantification expectations rise, theranostics links diagnostics with treatment, and data governance requirements grow more stringent. The most meaningful changes are not limited to detector performance; they involve how platforms support standardized workflows, produce comparable biomarkers, integrate securely with enterprise systems, and remain upgradeable as software and tracer ecosystems evolve.Tariff uncertainty in the United States adds urgency to disciplined procurement and lifecycle planning, pushing both buyers and vendors to emphasize supply-chain transparency, service predictability, and contingency readiness. Meanwhile, segmentation patterns show that modality choices and upgrade strategies are increasingly driven by operational throughput, staff efficiency, and application-specific needs across oncology, neurology, and cardiology. Regional differences further reinforce that no single deployment model fits all, and that service infrastructure, tracer access, and reimbursement conditions can be as decisive as technical specifications.
Organizations that treat molecular imaging as a strategic capability-governed, standardized, interoperable, and aligned to clinical pathways-will be best positioned to deliver consistent patient impact and sustain performance amid rapid technological and policy change.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Clinical Molecular Imaging System Market
Companies Mentioned
The key companies profiled in this Clinical Molecular Imaging System market report include:- Analogic Corporation
- Aspect Imaging Ltd.
- Bioscan, Inc.
- Bruker Corporation
- Canon Medical Systems Corporation
- Carestream Health, Inc.
- CMR Naviscan Corporation
- Cubresa Inc.
- Esaote S.p.A.
- Fujifilm Holdings Corporation
- GE Healthcare
- Hitachi, Ltd.
- Hologic, Inc.
- Koninklijke Philips N.V.
- Mediso Ltd.
- MILabs B.V.
- Mindray Bio-Medical Electronics Co., Ltd.
- PerkinElmer, Inc.
- PicoTools GmbH
- Samsung Medison Co., Ltd.
- Shimadzu Corporation
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
- Trivitron Healthcare
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
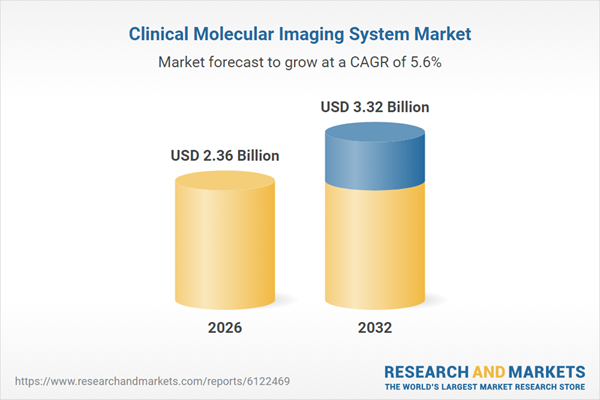
| Estimated Market Value ( USD | $ 2.36 Billion |
| Forecasted Market Value ( USD | $ 3.32 Billion |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |