Speak directly to the analyst to clarify any post sales queries you may have.
Setting the stage for wound care biologics as regenerative solutions amid rising chronic wound burden and tighter expectations on outcomes
Wound care biologics are redefining what “healing” means in complex and chronic wounds by shifting the clinical goal from temporary coverage to active regeneration. As the burden of diabetes, vascular disease, immobility, and post-surgical complications persists globally, clinicians and health systems are increasingly judged on measurable endpoints such as time to closure, infection avoidance, and recurrence reduction. In that environment, biologic solutions-ranging from collagen matrices to cellular and tissue-based products-have moved from niche adjuncts to integral tools within advanced wound protocols.At the same time, adoption is no longer driven solely by promising mechanisms of action. Procurement teams, payers, and provider networks are asking more pointed questions about standardized indications, reproducible outcomes across sites, and transparent product handling requirements. This has intensified the focus on evidence quality, clinician training, and the operational realities of storage, shelf life, and application technique.
Against this backdrop, the wound care biologics landscape is entering a period where scientific innovation must be paired with commercial discipline. Companies that can align product design with care pathways, reimbursement rules, and real-world workflow constraints are positioned to outperform-especially as hospitals and outpatient centers push for consistent results across diverse patient populations.
How clinical pathways, targeted innovation, and value-based scrutiny are transforming wound care biologics from products into protocol enablers
The landscape is undergoing a transformative shift as wound management evolves from episodic treatment to longitudinal care coordination. Provider systems are increasingly building standardized wound pathways that connect inpatient, outpatient, and home health settings, and biologics are being evaluated as part of an integrated protocol rather than a standalone purchase. This change favors products with clear patient selection guidance, repeatable application steps, and evidence that translates across care sites.In parallel, innovation is moving beyond “one-size-fits-all” matrices toward more targeted approaches that account for wound etiology, biofilm burden, and local tissue perfusion. Developers are investing in biologics that better manage inflammation, support granulation, and create a favorable microenvironment for closure. This is reinforced by a growing emphasis on combination strategies, where biologic scaffolds may be paired with negative pressure wound therapy, antimicrobial dressings, or debridement protocols to improve the probability of sustained healing.
Operational and regulatory expectations are also reshaping the competitive field. Traceability, donor screening documentation, chain-of-custody controls, and post-market surveillance have become more visible differentiators, especially for tissue-derived offerings. Meanwhile, value-based care pressures are pushing stakeholders to look beyond acquisition cost and toward avoided complications, fewer clinic visits, and lower downstream utilization. As a result, market participants are increasingly competing on total episode-of-care performance, not just product attributes.
Finally, digitization is subtly changing how biologics are selected and monitored. Wound imaging, documentation platforms, and analytics are making it easier to compare outcomes across clinicians and sites, which raises the bar for consistency. Companies that support this shift with training, clinical support, and evidence packages aligned to data-driven decision-making are gaining traction as partners rather than vendors.
Why United States tariffs in 2025 may reshape sourcing, pricing discipline, and operational resilience across wound care biologics supply chains
United States tariffs anticipated for 2025 introduce a cumulative impact that reaches far beyond a simple increase in landed costs. Wound care biologics rely on intricate global supply chains for single-use kits, sterile packaging, cold-chain components, and specialized processing inputs. Even when the biologic itself is sourced domestically, tariffs affecting upstream materials can raise total manufacturing costs and compress margins, particularly for products sold into price-sensitive outpatient settings.One immediate consequence is a renewed focus on supplier diversification and dual-sourcing strategies. Manufacturers are reevaluating dependency on single geographies for critical components such as medical-grade polymers, barrier packaging, and sterilization consumables. This shift tends to favor companies with mature procurement organizations and the ability to qualify alternate suppliers without compromising biocompatibility, sterility assurance, or shelf-life performance.
Tariff-driven cost pressure also accelerates conversations between manufacturers and provider organizations about contracting models. As health systems push to contain episode costs, vendors may need to justify any price movement with stronger outcomes narratives, more robust training support, and clearer economic value propositions. In certain cases, companies may respond by adjusting product configurations, optimizing kit contents, or streamlining SKUs to reduce exposure to tariff-impacted inputs while preserving clinical utility.
Over time, the cumulative effect may reshape investment priorities toward more localized manufacturing and packaging operations. While onshoring is not universally feasible-especially for specialized processing or donor tissue logistics-it can reduce exposure to trade volatility and improve responsiveness to demand fluctuations. In addition, regulatory strategy becomes intertwined with operations: changes in manufacturing location or suppliers can trigger validation work, documentation updates, and timeline risk. Organizations that plan early, model scenarios, and coordinate regulatory, quality, and sourcing teams will be better positioned to maintain continuity of supply and protect customer trust.
Segmentation signals that product selection now depends on wound etiology, care setting constraints, and evidence strength across biologic categories
Segmentation dynamics in wound care biologics reveal that product choice is increasingly governed by clinical context and operational practicality rather than brand familiarity alone. Across product types such as skin substitutes and grafts, collagen-based matrices, growth factor and biologic signaling products, and amniotic and placental-derived offerings, differentiation is becoming clearer around handling requirements, integration with standard wound bed preparation, and the strength of supporting evidence. In practice, clinicians often match these options to wound depth, exudate level, infection risk, and the need for structural scaffolding versus biochemical signaling.When viewed through the lens of wound type, diabetic foot ulcers, venous leg ulcers, pressure ulcers, and surgical wounds each exert distinct demands on biologic performance and utilization patterns. Diabetic foot ulcers frequently bring ischemia, neuropathy, and high infection risk, which makes careful patient selection and adjunctive offloading central to whether a biologic can deliver durable closure. Venous leg ulcers place more weight on compression adherence and management of edema, shaping the role of biologics as part of a broader regimen. Pressure ulcers underscore the importance of pressure redistribution and nutrition, where biologics can support granulation but cannot substitute for systemic and mechanical interventions. Surgical wounds and dehisced incisions often highlight the need for rapid coverage and reduced complication risk, with biologics used to improve healing trajectories when standard closure is insufficient.
Application setting segmentation further clarifies adoption. Hospitals may prioritize products that fit perioperative workflows and infection-control protocols, while outpatient wound care centers often emphasize repeatable application, reimbursement alignment, and inventory simplicity. Home healthcare introduces additional constraints, including patient adherence, limited procedural infrastructure, and the need for products that are easier to store and apply with predictable results. Meanwhile, long-term care environments frequently face staffing variability and high comorbidity burdens, increasing the value of protocols and training support that can standardize care.
End-user segmentation across clinicians, ambulatory centers, and integrated delivery networks indicates that purchasing influence is shifting toward committees that weigh clinical outcomes alongside operational impact. Surgeons, podiatrists, and wound specialists remain critical champions, yet materials management, pharmacy and therapeutics groups, and value-analysis teams increasingly shape formularies. Finally, distribution channel segmentation-spanning direct sales, specialty distributors, and group purchasing arrangements-highlights the importance of contracting agility. Companies that can align contracting terms with provider needs while supporting education and documentation requirements are better positioned to sustain utilization in environments where compliance and audit readiness are becoming routine.
Regional adoption of wound care biologics reflects infrastructure readiness, reimbursement realities, and specialist access across global care pathways
Regional dynamics show that adoption patterns in wound care biologics are shaped by infrastructure maturity, reimbursement architecture, and clinician access to advanced wound services. In the Americas, the concentration of specialized wound centers, established coding pathways, and high chronic disease prevalence supports broad use, yet scrutiny around documentation, repeat utilization, and episode-of-care value continues to intensify. Provider consolidation also influences contracting, making enterprise-wide standardization a growing requirement for vendors seeking scalable relationships.Across Europe, Middle East & Africa, the landscape is heterogeneous, with advanced capabilities in parts of Western Europe contrasted by variable access and budget constraints in other areas. Regulatory requirements and procurement models can favor suppliers that provide robust quality documentation and cost-effectiveness narratives suitable for tender processes. In the Middle East, expanding hospital infrastructure and specialty services support adoption, while parts of Africa often prioritize foundational wound management resources; biologics may be concentrated in tertiary centers where advanced care pathways exist.
Asia-Pacific presents a mix of high-growth potential and operational complexity. Large patient populations, rising diabetes incidence, and increasing investment in hospital modernization create favorable conditions for advanced wound therapies, particularly in major urban centers. However, access can be uneven across geographies, and differences in reimbursement, clinician training, and distribution capabilities can slow diffusion outside leading facilities. For companies, success often depends on pairing clinical education with reliable logistics and localized partnerships that support consistent product availability.
Across all regions, an important throughline is the growing role of guideline-driven care and measurable outcomes reporting. Regions that accelerate adoption tend to be those where wound care specialization is formalized, referral pathways are clear, and stakeholders can track healing progress with consistent documentation. Consequently, vendors that can support implementation-through training, protocol alignment, and post-application monitoring tools-are better positioned to expand use beyond early adopters.
Competitive advantage increasingly favors biologics companies that pair clinical evidence with dependable supply, training depth, and workflow integration
Company positioning in wound care biologics increasingly hinges on three pillars: clinical credibility, operational reliability, and the ability to integrate into real-world care pathways. Established participants tend to emphasize breadth of portfolio-covering matrices, tissue-based solutions, and adjunctive advanced dressings-so clinicians can select the most appropriate tool without changing vendors. This portfolio approach also supports contracting leverage with integrated delivery networks that prefer vendor rationalization.Innovators and focused specialists, by contrast, often compete through differentiated processing methods, clearer handling characteristics, or novel biologic compositions intended to improve consistency. Their success frequently depends on targeted clinical education and strong field support to ensure appropriate application, particularly when outcomes are sensitive to wound bed preparation, debridement timing, or offloading compliance.
Across the competitive set, the quality of evidence and post-market support has become more central to sustaining utilization. Companies that invest in pragmatic studies, registries, and real-world documentation support can better address payer and value-analysis scrutiny. Additionally, reliable fulfillment and lot-to-lot consistency matter more than ever; stockouts or variability can disrupt protocols and quickly erode clinician confidence.
Partnerships are also shaping the field. Collaboration with wound imaging platforms, outpatient networks, and distribution specialists helps companies embed biologics into standardized workflows. At the same time, manufacturing and sourcing partnerships-particularly around sterilization capacity and specialized packaging-are becoming strategic differentiators as supply resilience moves up the agenda.
Practical recommendations to win in wound care biologics by reinforcing protocols, de-risking supply chains, and proving value in daily workflows
Industry leaders can strengthen their position by designing strategies around the full episode of care rather than single-product adoption. This starts with sharpening patient selection and protocol guidance so clinicians know when a biologic is most likely to succeed and how to sequence it with debridement, infection management, compression, or offloading. Clear decision tools and standardized documentation templates can reduce variation and support audit readiness.Next, organizations should harden supply chain resilience in anticipation of tariff volatility and broader trade disruption. Prioritizing dual-sourcing for tariff-exposed inputs, qualifying alternate packaging and kit components, and building contingency inventory policies can protect continuity. When manufacturing or supplier changes are required, early coordination between regulatory, quality, and operations teams reduces validation risk and prevents downstream delays.
Commercially, leaders should align contracting and clinical support to the realities of hospitals, outpatient wound centers, and home health. In settings where staff turnover is high, structured training and certification-style education can preserve application consistency. Where payer scrutiny is strong, a disciplined evidence package that links product use to practical outcomes-fewer complications, fewer visits, and sustained closure-can help defend utilization without relying on price concessions.
Finally, companies should invest in implementation support that makes adoption easier. Integration with documentation workflows, compatibility with imaging and measurement tools, and responsive clinical education create stickier relationships. Over time, leaders that become trusted partners in protocol deployment-rather than intermittent suppliers-will be better positioned to expand across sites within consolidated health systems.
A transparent research methodology combining stakeholder validation, regulatory and clinical review, and triangulated synthesis for decision-ready insights
This research methodology is structured to capture how wound care biologics perform within clinical practice, procurement environments, and regulatory constraints. The approach begins with a comprehensive mapping of the product landscape, including biologic categories, processing approaches, and typical use cases across chronic and acute wound etiologies. This is complemented by a review of regulatory frameworks and quality expectations that influence commercialization and adoption.Primary research inputs are derived from structured interactions with stakeholders across the ecosystem, including clinicians involved in wound management, administrators responsible for wound programs, procurement and value-analysis participants, and industry experts familiar with manufacturing and distribution. These discussions are used to validate workflow realities, identify adoption barriers, and understand decision criteria such as handling, training burden, and documentation requirements.
Secondary research synthesizes publicly available sources such as regulatory databases, company filings, product instructions for use, peer-reviewed clinical literature, clinical guideline statements, and procurement and reimbursement policy documents. Triangulation is applied to reconcile differences across sources, while a consistency check process is used to confirm that insights align with current clinical practice and policy direction.
Finally, insights are organized using a structured framework that connects segmentation, regional dynamics, and competitive positioning. This ensures findings remain actionable for decision-makers who must translate clinical evidence and operational constraints into portfolio, partnering, and go-to-market choices.
Closing perspective on wound care biologics as protocols, evidence standards, and supply resilience determine long-term clinical and commercial success
Wound care biologics are entering a more disciplined era in which success depends on repeatable outcomes, operational fit, and credible evidence that resonates with both clinicians and value-focused decision-makers. As care pathways become more standardized and data visibility improves, products that cannot demonstrate consistent performance-or that are difficult to handle within real workflows-will face increasing friction regardless of theoretical benefit.Simultaneously, external pressures such as tariff-related cost volatility and supply chain fragility are elevating resilience as a competitive requirement. The strongest players will be those that anticipate disruption, protect continuity of supply, and maintain trust through dependable service and documentation support.
Looking ahead, the most durable growth opportunities will come from aligning biologics with protocol-driven care, enabling clinician confidence through training and evidence, and supporting health systems as they measure value across the full wound healing journey. Companies that operationalize these priorities will be best positioned to convert scientific promise into sustained clinical adoption.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Wound Care Biologics Market
Companies Mentioned
The key companies profiled in this Wound Care Biologics market report include:- 3M Company
- Aroa Biosurgery
- Avita Medical
- B. Braun SE
- Baxter International Inc.
- Bioventus Inc.
- Cardinal Health
- Celularity Inc.
- CollPlant Biotechnologies Ltd.
- Coloplast A/S
- Convatec Group Plc
- DeRoyal Industries, Inc.
- Essity Health & Medical
- Grifols
- Hollister Incorporated
- Integra LifeSciences Holdings Corporation
- Johnson & Johnson Services, Inc.
- Kerecis Ehf.
- Medline Industries, LP
- Medtronic PLC
- MiMedx Group, Inc.
- Mölnlycke Health Care AB
- Organogenesis Holdings Inc.
- Smith & Nephew Plc
- Stryker Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
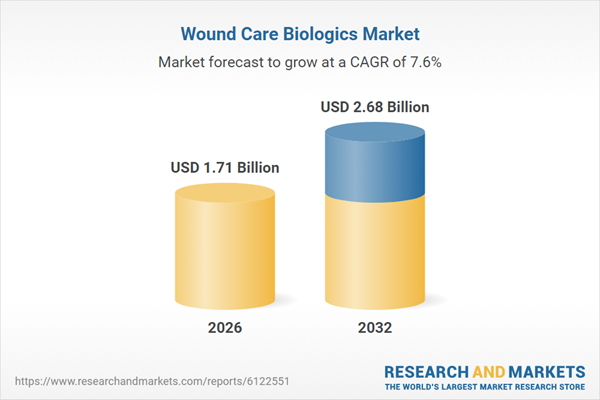
| Estimated Market Value ( USD | $ 1.71 Billion |
| Forecasted Market Value ( USD | $ 2.68 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |