Speak directly to the analyst to clarify any post sales queries you may have.
Blood lipid test kits are becoming a cornerstone of preventive cardiometabolic care as accuracy, speed, and accessibility converge
Blood lipid test kits sit at the intersection of cardiometabolic screening, preventive medicine, and the expanding expectation that diagnostics travel with the patient. Lipid panels-commonly including total cholesterol, HDL, LDL (direct or calculated), non-HDL cholesterol, and triglycerides-remain foundational for cardiovascular risk assessment and therapy monitoring. As care pathways shift toward earlier detection and tighter follow-up, the value of dependable, reproducible lipid results has increased for clinicians, payers, and consumers alike.Demand is being shaped by three converging forces. First, a growing global burden of dyslipidemia and related conditions is sustaining routine lipid monitoring across primary care and specialist cardiology. Second, operational pressure on clinics and laboratories is strengthening the case for rapid, workflow-friendly solutions that minimize sample handling and shorten turnaround time. Third, self-care and retail health models are normalizing patient-driven testing behaviors, provided results can be trusted and, ideally, shared with clinicians.
Against this backdrop, blood lipid test kits span a range of use cases from centralized laboratory workflows to near-patient and home settings. Performance expectations vary by setting, but the market is broadly unified by the same priorities: analytical accuracy, lot-to-lot consistency, robust quality controls, clear labeling and instructions, and regulatory compliance. The executive summary that follows frames how technology, policy, and commercialization strategies are evolving, and what decision-makers should watch when positioning products, building partnerships, or modernizing testing programs.
The market is shifting toward connected, multi-setting lipid testing where usability, interoperability, and quality systems drive adoption
The landscape is undergoing a decisive shift from single-channel laboratory procurement to multi-channel diagnostic ecosystems. Traditional lab-based testing remains essential, yet providers are increasingly complementing centralized panels with point-of-care options that support immediate counseling and therapy adjustments. In parallel, consumer-focused testing is moving beyond novelty toward structured programs tied to coaching, pharmacy-based services, and longitudinal health records. This shift is elevating expectations for standardization across settings so that results remain clinically meaningful when a patient transitions between a clinic, a retail site, and home.Technologically, the market is moving toward higher integration and better usability. Kit designs are increasingly optimized for smaller sample volumes, simplified preparation steps, and reduced operator variability. For point-of-care contexts, portability and resilience to variable environmental conditions are becoming more important, particularly for outreach screening and resource-limited sites. At the same time, laboratory environments are seeing continued emphasis on automation compatibility, improved reagent stability, and traceability features that support compliance and audits.
Data connectivity is another major transformation. Stakeholders want lipid results to flow into electronic health records, remote monitoring dashboards, and population health analytics without manual transcription. As a result, solutions that align with interoperability expectations and offer clean interfaces to existing systems are gaining an advantage. This is also influencing kit selection: purchasers are weighing not only analytical metrics but also how results are captured, stored, and shared.
Regulatory and quality expectations are tightening in tandem. Authorities and accreditation bodies increasingly scrutinize labeling claims, clinical performance evidence, and post-market surveillance. Quality management maturity-spanning documentation, complaint handling, change control, and supplier qualification-is becoming a competitive differentiator. Consequently, manufacturers that can demonstrate consistent performance across lots and geographies, while maintaining responsive technical support, are better positioned to win long-cycle tenders and maintain durable customer trust.
United States tariff dynamics in 2025 are reshaping sourcing, contracting, and continuity planning across blood lipid test kit supply chains
The 2025 tariff environment in the United States is expected to influence blood lipid test kits through cost structure, sourcing strategy, and contracting dynamics rather than through a single uniform outcome. Because kits often depend on globally sourced components-such as specialized plastics, antibodies or enzymes, calibrators, control materials, and analyzer-compatible consumables-tariffs can affect multiple tiers of the bill of materials. Even when the final kit is assembled domestically, upstream inputs may still experience cost pressure that flows through to pricing discussions.One of the most immediate impacts is procurement volatility. Hospitals, integrated delivery networks, and reference laboratories typically lock pricing through contracts, but tariff-driven changes can trigger renegotiations, surcharges, or shifts in preferred suppliers. This environment favors manufacturers that can offer transparent cost narratives, stable fulfillment, and contingency inventory plans. Conversely, suppliers with narrow sourcing options may face margin compression or service-level risks, especially when demand spikes seasonally due to preventive screening campaigns.
Tariffs also accelerate strategic localization decisions. Some manufacturers will reassess whether to dual-source critical components, qualify alternative suppliers, or expand domestic assembly and packaging capacity to reduce exposure. However, requalification is not trivial in diagnostics: changes to materials, reagents, or manufacturing sites can require additional verification, stability work, and documentation to protect performance claims. Therefore, organizations that already invested in modular designs and disciplined change management can adapt faster without disrupting customers.
Finally, the tariff backdrop is reshaping how buyers evaluate total value. When price variability increases, purchasers often place more weight on reliability, shelf life, technical support, and quality documentation, because operational disruption can be more costly than incremental unit-price differences. In practice, this means that companies able to quantify continuity-of-supply advantages and demonstrate robust quality controls may improve their standing in competitive evaluations, even as the cost base shifts.
Segmentation shows distinct buying logic across kit formats, test approaches, end-use settings, and channels as lipid testing expands beyond labs
Segmentation reveals that the market behaves differently depending on how and where lipid testing is performed, and what clinical or consumer objective is being served. From a product perspective, kits designed for complete lipid profiling emphasize consistency across multiple analytes and strong calibration approaches, while more focused offerings prioritize convenience and speed for targeted screening. This distinction matters because workflow and interpretation needs differ: comprehensive panels often support ongoing disease management, whereas simpler configurations can be more aligned with rapid triage and outreach screening.When viewed through the lens of test type and analytical approach, enzymatic colorimetric methods remain common due to established performance and broad compatibility, yet there is an active push toward designs that reduce interference and improve precision at clinically important decision points. As programs place greater emphasis on repeat testing and therapy monitoring, buyers increasingly ask for evidence that results remain stable across operators, lots, and environmental conditions. This is particularly pronounced when point-of-care deployment is expanded beyond a single clinical site.
End-use and setting segmentation further clarifies purchasing drivers. Hospital laboratories and large reference labs often prioritize throughput, automation fit, and audit-ready documentation, while smaller clinics may value simplified handling, reduced training burden, and fast turnaround to support same-visit counseling. Home and self-testing contexts heighten the importance of clear instructions, integrated controls, and user-centric packaging that reduces the risk of pre-analytical errors. Across these settings, quality assurance is not optional; rather, the form it takes changes, with centralized sites leaning on formal QC programs and decentralized sites relying more heavily on built-in safeguards and guided workflows.
Channel and customer segmentation highlight another shift: procurement is increasingly hybrid. Traditional institutional purchasing remains influential, yet pharmacy-led health services, employer wellness programs, and digital health platforms are shaping new routes to market. These channels reward manufacturers that can deliver consistent training materials, scalable technical support, and data pathways that make results usable beyond a single encounter. In this environment, differentiation is less about a single feature and more about how the kit fits into a full service model, from sample collection and quality checks to actionable reporting.
Regional adoption patterns reflect differences in preventive care priorities, regulatory expectations, and infrastructure readiness across major geographies
Regional dynamics are defined by how health systems balance preventive screening with cost control, and by how regulatory maturity shapes product availability and claims. In the Americas, demand is strengthened by established cardiovascular prevention guidelines and a sizable installed base of laboratory infrastructure, while decentralization trends are expanding point-of-care placements in clinics and retail settings. Buyers frequently emphasize standardized performance, strong technical documentation, and dependable logistics, reflecting a procurement culture that favors continuity and compliance.In Europe, the market is influenced by structured preventive programs and strong expectations for quality management, labeling, and post-market oversight. Cross-country variation remains important: reimbursement approaches, tender structures, and care pathways differ, which affects how rapidly new kit designs are adopted. Nonetheless, solutions that support consistent results across multi-site networks and that streamline documentation for audits are particularly valued, as providers seek efficiency without compromising analytical integrity.
The Middle East and Africa present a mixed but increasingly important opportunity landscape. Gulf countries and selected urban centers are investing in modern laboratory capacity and preventive initiatives, while other areas prioritize scalable screening that can operate under variable conditions. In these settings, reagent stability, simplified workflows, and supplier-led training can be decisive. Partnerships that include service support and reliable supply planning often matter as much as product attributes.
Asia-Pacific combines high-volume potential with diverse regulatory and care delivery environments. Several markets are expanding chronic disease screening and strengthening primary care, which supports broader lipid testing adoption. At the same time, cost sensitivity and procurement complexity can be high, prompting interest in solutions that deliver strong value per test while maintaining quality. Manufacturers that localize support, adapt packaging and instructions for varied user contexts, and invest in distributor capability building tend to perform better across this region’s heterogeneous demand profiles.
Competitive advantage is consolidating around performance proof, workflow integration, and service-led partnerships that sustain adoption across settings
Company strategies in blood lipid test kits increasingly converge on three competitive priorities: performance credibility, workflow fit, and service enablement. Established diagnostics leaders leverage broad assay portfolios, analyzer ecosystems, and deep regulatory and quality infrastructure to secure long-term relationships with hospitals and reference laboratories. Their advantage often lies in demonstrated consistency, mature quality controls, and the ability to support complex procurement requirements, including multi-site standardization.At the same time, specialized and emerging players are carving out positions by focusing on ease of use, portability, and solutions designed for decentralized care. These companies often compete by simplifying sample handling, shortening time-to-result, and integrating guidance that reduces operator dependence. When paired with thoughtful connectivity options and training support, these approaches align well with clinics, retail health, and outreach programs seeking efficient screening at scale.
Across the competitive set, partnerships are becoming a primary mechanism for expansion. Collaborations with distribution networks, pharmacy chains, digital health platforms, and contract manufacturing organizations allow companies to reach new customer segments without building every capability in-house. Additionally, supplier relationships for critical reagents and consumables are being reassessed to improve resilience, particularly under shifting trade conditions and elevated scrutiny on continuity of supply.
Differentiation is increasingly proven in the field rather than asserted in marketing. Buyers scrutinize real-world performance consistency, complaint responsiveness, training effectiveness, and the clarity of instructions and labeling. As a result, companies that invest in customer success models-covering onboarding, quality program support, and post-market feedback loops-can strengthen retention and reduce the friction that often slows adoption in regulated diagnostic environments.
Leaders can outperform by building resilient supply chains, interoperability-first designs, and deployment models tailored to each care setting
Industry leaders can strengthen their position by treating lipid testing as an end-to-end experience rather than a stand-alone consumable. Prioritizing user-centered design-especially in decentralized settings-reduces pre-analytical and handling errors that undermine confidence in results. This includes clearer instructions, stronger on-kit or in-process controls, and packaging engineered for real-world storage and transport constraints.Supply chain resilience should be elevated to a strategic capability, particularly in light of tariff-driven uncertainty. Dual-sourcing critical inputs, maintaining disciplined supplier qualification, and building scenario-based inventory policies can help protect service levels. Equally important is cross-functional coordination so that cost changes, component substitutions, and manufacturing shifts are managed through robust change control with minimal disruption to customers and regulatory commitments.
Leaders should also invest in interoperability and evidence generation. Making results easier to capture and share increases clinical utility and supports integration into chronic care programs. In parallel, structured post-market data collection and customer feedback systems can demonstrate reliability in the environments where kits are actually used, reinforcing credibility in procurement decisions.
Commercially, companies can win by aligning offerings to specific care models. For hospitals and large laboratories, this may mean emphasizing standardization, documentation, and automation compatibility. For clinics and retail health, it may mean deployment toolkits, training at scale, and service-level commitments. For consumer programs, it may mean guided workflows and pathways for clinician follow-up. In all cases, positioning should translate analytical performance into operational outcomes-reduced retesting, smoother workflows, and more actionable conversations with patients.
A rigorous mixed-method research approach connects stakeholder interviews with regulatory and industry validation to deliver practical insights
This research uses a structured methodology designed to translate complex diagnostic markets into decision-ready insights. The work begins with a comprehensive mapping of blood lipid test kit applications, including laboratory-based testing, near-patient workflows, and consumer-oriented use cases. This framing ensures that product attributes are evaluated in the context of real operational needs, regulatory constraints, and clinical decision pathways.Primary research incorporates qualitative engagement with stakeholders across the value chain, such as manufacturers, distributors, laboratory professionals, clinicians, and procurement participants. These conversations focus on purchasing criteria, workflow bottlenecks, quality expectations, and the practical implications of policy and trade changes. Insights are cross-checked across multiple interviews to reduce single-source bias and to surface where perspectives diverge by setting or region.
Secondary research includes review of publicly available regulatory information, standards and guidance documents, company materials, and credible industry publications. This stage supports validation of technology claims, identification of compliance considerations, and understanding of competitive positioning. Throughout the process, attention is given to avoiding over-reliance on any one narrative by triangulating across different document types and stakeholder viewpoints.
Finally, findings are synthesized using an analytical framework that connects product design and performance requirements to commercialization realities such as distribution models, service expectations, and supply chain risk. The result is an integrated view that helps decision-makers assess strategic options, prioritize investments, and anticipate implementation challenges when deploying or expanding blood lipid testing programs.
The market is converging on trustworthy, connected, and resilient lipid testing solutions as care models decentralize and scrutiny rises
Blood lipid test kits are evolving from routine lab supplies into strategic enablers of preventive care and chronic disease management across diverse settings. As testing expands beyond centralized laboratories, stakeholders are demanding solutions that preserve analytical credibility while improving usability, connectivity, and operational efficiency. The most successful offerings will be those that support consistent outcomes regardless of where the test is performed.At the same time, policy and supply chain dynamics are increasing the premium placed on resilience. Tariff-related uncertainty and broader procurement volatility are pushing both manufacturers and buyers to rethink sourcing, contracting, and continuity planning. This environment rewards companies that can demonstrate disciplined quality systems, transparent supply strategies, and dependable service.
Taken together, the market’s direction is clear: value is shifting toward solutions that combine trustworthy performance with ecosystem fit. Decision-makers who align product strategy, partnerships, and operational readiness to these realities will be better positioned to support clinicians and consumers while navigating regulatory expectations and competitive pressure.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Blood Lipid Test Kits Market
Companies Mentioned
The key companies profiled in this Blood Lipid Test Kits market report include:- Abbott Laboratories
- Alfresa Holdings Corporation
- ARKRAY, Inc.
- Asahi Kasei Corporation
- Beckman Coulter, Inc.
- bioMérieux SA
- Daiichi Sankyo Company, Limited
- Denka Seiken Co., Ltd.
- Eiken Chemical Co., Ltd.
- F. Hoffmann-La Roche AG
- Fujifilm Wako Shibayagi Corporation
- Horiba, Ltd.
- Kyowa Medex Co., Ltd.
- Mitsubishi Chemical Medience Corporation
- Nitto Boseki Co., Ltd.
- Ortho Clinical Diagnostics
- Randox Laboratories Ltd.
- Sekisui Medical Co., Ltd.
- Shino-Test Corporation
- Siemens Healthineers AG
- SRL, Inc.
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- Toyobo Co., Ltd.
- Wako Pure Chemical Industries, Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | January 2026 |
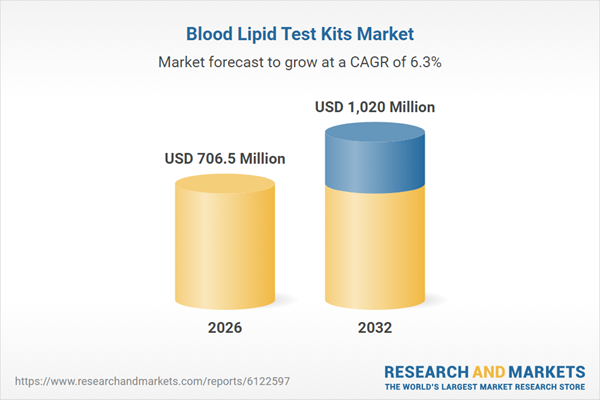
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 706.5 Million |
| Forecasted Market Value ( USD | $ 1020 Million |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |