Speak directly to the analyst to clarify any post sales queries you may have.
A strategic orientation to mobile C-arm imaging that synthesizes technological trajectories, clinical imperatives, and purchaser decision levers for confident leadership choices
The mobile C-arm X-ray arena sits at the intersection of imaging innovation and procedural care, where device mobility, image clarity, and workflow efficiency shape clinical outcomes across diverse procedural settings. Advances in detector technology, software-enabled imaging, and ergonomic design are redefining expectations for intraoperative visualization while shifting the conversation from basic radiography toward integrated imaging ecosystems. In parallel, evolving clinical pathways and an emphasis on minimally invasive interventions have elevated the strategic importance of nimble imaging platforms that deliver consistent imaging performance without compromising throughput.As hospitals and ambulatory settings rethink capital allocation, key stakeholders require concise, high-fidelity intelligence to understand product differentiation, interoperability considerations, and service and lifecycle economics. The landscape also reflects regulatory nuance and supply chain dynamics that influence procurement timelines and total cost of ownership. Therefore, a grounded introduction to this market must frame technological trajectories, clinical drivers, and purchaser imperatives in a manner that supports evidence-based decisions and operational resilience.
How detector modernization, software integration, and procedural migration are reshaping product design priorities and commercial strategies across mobile C-arm ecosystems
The landscape for mobile C-arm systems is transforming as several converging forces recalibrate product road maps and purchasing behavior. Digitization and the rise of flat panel detectors have accelerated a transition away from legacy image intensifiers toward systems that deliver higher resolution, lower dose, and enhanced post-processing capabilities. Concurrently, software advances such as AI-enabled noise reduction, image stitching, and real-time dose monitoring are shifting value from hardware alone to integrated imaging platforms that enable procedural guidance and documentation.At the same time, procedural migration into ambulatory surgical centers and hybrid OR environments has altered form factor and portability requirements, prompting vendors to re-evaluate weight, maneuverability, and sterilizable interfaces. Value-based procurement and lifecycle service models are encouraging manufacturers to bundle software upgrades and predictive maintenance features to sustain clinical reliability. Moreover, supply chain resilience and component sourcing strategies are influencing design choices, particularly with respect to detector availability and custom ASIC dependencies. As a result, both incumbents and new entrants are prioritizing modularity and software-centric differentiation to capture broader clinical workflows and to secure long-term installed base relationships.
Assessing the layered supply chain, procurement, and compliance consequences of 2025 United States tariff actions on mobile C-arm manufacturing and purchasing
The introduction of tariff measures and trade policy shifts emanating from the United States in 2025 created layered implications for the mobile C-arm supply chain, procurement strategies, and capital equipment economics. Increased duties on certain medical device components prompted immediate supplier reassessment and triggered near-term sourcing adjustments to mitigate input-cost volatility. Procurement teams responded by re-evaluating vendor contracts, renegotiating terms, and exploring alternative component suppliers to preserve margin and delivery schedules. In several cases, original equipment manufacturers repositioned assembly footprints or deepened relationships with regional suppliers to reduce exposure to cross-border tariff risk.Beyond immediate cost pressures, tariffs amplified strategic conversations around localization and compliance. Manufacturers accelerated qualification of domestically sourced components and invested in dual-sourcing strategies for critical subsystems such as detectors, X-ray tubes, and power electronics. This shift required more robust supplier governance and longer validation timelines, affecting product launch cadence and aftermarket availability. In parallel, clinical customers experienced elongated procurement lead times and more complex total cost deliberations that extended beyond sticker price to include logistics complexity, customs clearance variability, and warranty servicing implications.
Furthermore, tariff-related uncertainty influenced capital allocation behavior among healthcare providers. Some systems deferred non-urgent purchases to reassess vendor risk profiles, while others prioritized models with established regional supply chains or with service agreements that absorbed some logistical risk. For manufacturers, the tariff environment heightened focus on price-to-value narratives, pushing product teams to emphasize reliability, uptime guarantees, and bundled software services that rationalize higher acquisition spend through predictable lifecycle economics.
Importantly, the cumulative impact of tariffs also catalyzed industry-wide emphasis on policy monitoring and trade compliance capabilities. Risk management functions expanded to include scenario analysis for tariff fluctuations and to model supply chain exposure by component category. Legal and regulatory teams increased engagement with customs authorities and industry associations to seek clarity and to advocate for harmonized tariff treatment for essential medical equipment. Collectively, these responses underscore how trade policy shifts in 2025 prompted tactical adjustments and longer-term strategic realignment across the mobile C-arm value chain.
How layered segmentation across clinical application, product typology, end-user environment, imaging technology, and detector architecture informs differentiated commercialization strategies
Segmentation-driven insights reveal how clinical applications, product types, end-user settings, technological platforms, and detector architectures each create distinct adoption patterns and investment priorities. Based on Application, the market is studied across Cardiovascular, Gastroenterology, Orthopedics, Pain Management, and Urology, which underscores the modality’s cross-specialty utility and the need for configurable imaging presets and tailored workflows. Based on Product Type, the market is studied across General Purpose C Arm, Hybrid C Arm, and Mini C Arm, with the Hybrid C Arm further studied across Three D Hybrid C Arm and Two D Hybrid C Arm, highlighting the spectrum from compact mobility to high-end intraoperative navigation capability. Based on End User, the market is studied across Ambulatory Surgical Centers, Clinics, Diagnostic Centers, and Hospitals, reflecting divergent purchasing models, throughput expectations, and technical staffing levels that shape service agreements and user training requirements. Based on Technology, the market is studied across Analog and Digital, noting the persistent tail of legacy systems in lower-resource settings alongside rapid digital conversion in high-volume procedural environments. Based on Detector Type, the market is studied across Flat Panel Detector and Image Intensifier, which demonstrates a clear technological bifurcation in image quality, dose efficiency, and post-processing potential.Integrating these segmentation lenses reveals practical implications for product planning and commercial strategy. Clinical applications such as orthopedics and cardiovascular interventions typically demand higher-resolution imaging and predictable C-arm maneuverability, which informs choices around detector type and system rigidity. Conversely, ambulatory and clinic settings often prioritize compact footprints and rapid setup times, favoring mini C-arm designs or general-purpose systems that optimize throughput and ease of sterilization. The rise of hybrid ORs and complex interventional suites drives investment in hybrid C-arm variants with three-dimensional capability, which necessitates higher vendor proficiency in integration with navigation and robotic platforms. Technology transitions from analog to digital underscore upgrade pathways and aftermarket software monetization opportunities, while detector selection continues to delineate performance tiers and lifetime service considerations. Therefore, segmentation is not merely classificatory; it directly translates into differentiated product road maps, tailored service packages, and bespoke channel strategies that align with specific clinical and operational requirements.
Regional dynamics that dictate procurement behavior, regulatory pathways, and aftermarket strategies across the Americas, Europe Middle East & Africa, and Asia-Pacific markets
Regional dynamics exert a profound influence on procurement practices, regulatory navigation, and innovation diffusion for mobile C-arm systems. In the Americas, demand patterns reflect a blend of high-volume tertiary centers and a mature ambulatory surgical network that emphasizes procedural efficiency, robust service frameworks, and integration readiness with electronic health records and intraoperative data systems. Transitioning to Europe, Middle East & Africa, regulatory diversity and reimbursement heterogeneity create both complexity and opportunity; suppliers must navigate strict conformity pathways while tailoring financing and service models for regions with constrained capital budgets. Across Asia-Pacific, rapid infrastructure investment, expanding surgical volumes, and rising availability of skilled clinical operators are driving adoption of digital imaging platforms, yet supply chain localization and component qualification remain central to sustained growth.These geographic nuances also influence after-sales models and clinical education programs. In regions with concentrated tertiary centers, vendors prioritize onsite training, high-availability service contracts, and advanced applications support. In contrast, markets with distributed clinic networks place greater emphasis on remote diagnostics, modular servicing solutions, and simplified user interfaces that reduce dependency on specialized technicians. Additionally, regulatory and import frameworks in each region impact lead times and product variant certification, which in turn shapes inventory strategies, regional warehousing, and channel partner selection. Consequently, a geographically informed commercial approach combines localized product configurations with adaptive service architectures and regulatory foresight to align with divergent healthcare delivery models.
Competitive strategies driven by detector and software innovation, aftermarket service excellence, and ecosystem partnerships that enhance clinical integration and commercial resilience
Competitive dynamics in the mobile C-arm space center on product differentiation, service excellence, and ecosystem partnerships that extend beyond equipment sales into software and data services. Leading manufacturers invest in detector innovation, algorithmic image enhancement, and ergonomics to create perceptible gains in clinical workflow and diagnostic confidence. At the same time, several firms strengthen aftermarket capabilities by offering predictive maintenance, remote diagnostics, and outcome-linked service agreements that align vendor incentives with clinical uptime and patient throughput.Strategic collaboration is increasingly common, as device makers partner with software developers, navigation providers, and hospital systems to ensure seamless interoperability and to validate clinical efficacy for advanced applications. This trend also includes collaboration with component suppliers to secure supply continuity and to accelerate qualification of next-generation detectors and ASICs. Moreover, some companies pursue modular architectures that enable incremental upgrades-such as detector swaps or software feature packs-thereby reducing the friction of capital refresh cycles and creating recurring revenue opportunities.
Finally, M&A and strategic investments play a role in consolidating expertise and fast-tracking access to complementary technologies. Acquisitions focused on software, imaging algorithms, or service platforms allow hardware-centric firms to move up the value chain and to offer integrated solutions that address the evolving needs of hybrid ORs and ambulatory surgery providers. As clinical demand shifts toward image-guided and minimally invasive procedures, companies that can deliver cohesive imaging ecosystems with robust service commitments will command competitive advantage.
Actionable playbook for manufacturers and healthcare leaders to secure supply continuity, monetize software-enabled services, and accelerate clinical adoption in varied care settings
Industry leaders should adopt a proactive posture that balances short-term supply continuity with long-term product and service differentiation. First, they should prioritize supplier diversification and component qualification pathways to mitigate trade policy and logistics disruption, thereby safeguarding production schedules and aftermarket service levels. In parallel, investing in modular system architectures and software-upgradable platforms enables more flexible product lifecycles and offers customers predictable upgrade paths that reduce total ownership friction.Second, companies should intensify efforts on software-enabled value propositions-including AI-based image enhancement, dose management, and workflow automation-that shift value capture toward recurring revenue and closer clinical integration. Aligning commercial models to emphasize outcome-linked service agreements and uptime guarantees will resonate with procurement teams focused on predictable operational economics. Third, manufacturers and channel partners should deepen clinical training and remote support capabilities to accelerate adoption curves in decentralized care environments. By combining on-site competency development with advanced remote troubleshooting, vendors can reduce downtime and increase clinician confidence.
Fourth, senior leaders must incorporate regulatory and trade scenario planning into portfolio decision-making, ensuring that market-entry strategies and pricing frameworks reflect regional compliance realities. Finally, forging targeted alliances with hospital systems and software providers will expedite the adoption of hybrid and 3D imaging workflows, while strategic investments in localized manufacturing or assembly can insulate operations from tariff-driven volatility. Collectively, these actions enable market participants to protect margins, preserve service commitments, and capture long-term growth driven by evolving clinical practice.
Transparent methodology that combines stakeholder interviews, regulatory review, and scenario-based supply chain and technology analysis to produce actionable and auditable insights
This research synthesizes primary interviews with procurement leaders, clinical engineers, and senior product managers, combined with secondary analysis of regulatory guidance, technology white papers, and publicly available corporate disclosures to construct a multidimensional view of the mobile C-arm landscape. Data collection prioritized cross-functional inputs to ensure that clinical requirements, service delivery constraints, and manufacturing realities are represented with fidelity. Quality assurance involved triangulating vendor claims against clinical peer literature and device registration records to validate performance assertions and feature sets.Analytical frameworks encompassed segmentation mapping, supply chain exposure analysis, and scenario-based stress testing to explore the implications of trade policy shifts and technological adoption curves. Wherever applicable, comparative benchmarking assessed device ergonomics, detector capabilities, and software feature depth to highlight meaningful differentiation. The methodology also incorporated validation workshops with independent clinical advisors to refine practical implications and to ensure recommendations align with real-world procurement and operational constraints. Throughout, the approach emphasized transparency and auditability, documenting assumptions and data sources to enable confident interpretation and application by decision-makers.
Convergent implications for manufacturers and providers as detector, software, and supply chain dynamics converge to determine long-term clinical and commercial winners
Mobile C-arm imaging continues to evolve into a software-augmented, clinically adaptable platform that must reconcile the needs of varied procedural specialties with supply chain and regulatory realities. Detector modernization, software-enabled imaging enhancements, and the emergence of hybrid workflows are collectively reshaping product design priorities and aftermarket service models. These shifts mean that vendors focused solely on hardware differentiation risk commoditization unless they embed software capabilities, robust service offerings, and flexible upgrade pathways into their core propositions.For healthcare providers, decision-making will increasingly hinge on interoperability, ease of integration, and predictable lifecycle economics rather than acquisition price alone. Meanwhile, trade policy and component sourcing will remain salient factors in procurement risk assessment, prompting both manufacturers and buyers to adopt more rigorous supplier governance and scenario planning. Ultimately, the market rewards orchestration: companies that combine technological excellence with resilient supply chains, compelling service models, and close clinical partnerships will be best positioned to support evolving procedural care and to capture durable customer relationships.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Mobile C-Arm X-ray Machines Market
Companies Mentioned
The key companies profiled in this Mobile C-Arm X-ray Machines market report include:- Canon Inc
- Canon Medical Systems Corporation
- GE HealthCare Technologies, Inc.
- Hologic, Inc.
- Koninklijke Philips N.V.
- OrthoScan, Inc.
- Planmed Oy
- Samsung Medison Co., Ltd.
- Shimadzu Corporation
- Siemens Healthineers AG
- Ziehm Imaging GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
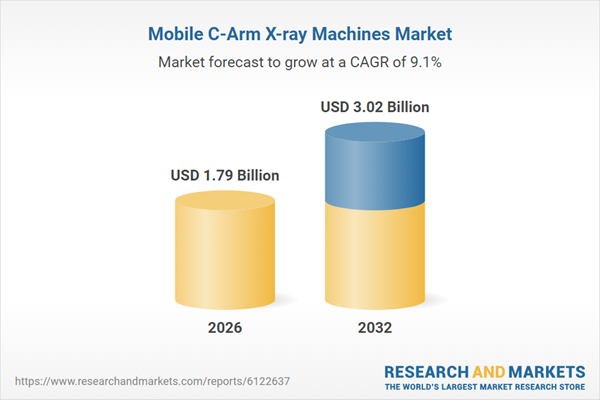
| Estimated Market Value ( USD | $ 1.79 Billion |
| Forecasted Market Value ( USD | $ 3.02 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |