Speak directly to the analyst to clarify any post sales queries you may have.
A concise authoritative synthesis framing scientific, regulatory, supply chain, and application intersections for androstenedione and derivative compounds
This executive summary synthesizes recent developments and strategic considerations surrounding androstenedione and its derivative compounds, offering an integrated perspective for industry leaders, clinicians, and research organizations. The objective is to articulate the scientific context, regulatory dynamics, supply chain nuances, and application-focused trends that are shaping decisions across cosmetics, supplements, pharmaceuticals, and laboratory research. By drawing connections across formulation types, derivative chemistries, distribution pathways, and end-user needs, the narrative highlights practical implications while preserving the technical rigour required by stakeholder audiences.Emerging scientific evidence, evolving regulatory interpretations, and shifts in commercial distribution models have collectively redefined risk and opportunity profiles. The content that follows distills these elements into actionable insights, emphasizing where incremental adjustments to product portfolios, sourcing strategies, and compliance frameworks can yield outsized strategic benefits. Readers can expect a concise yet comprehensive roadmap that supports short- and medium-term planning, harmonizes cross-functional priorities, and directs attention to critical inflection points.
Throughout, the analysis adopts a pragmatic lens: it acknowledges scientific complexity, anticipates regulatory evolution, and foregrounds commercial channels that affect accessibility and stewardship. This introduction sets the stage for more granular discussions on transformative shifts, tariff impacts, segmentation nuances, regional differentials, competitive positioning, recommended actions, and the methods employed to derive these conclusions.
How advances in analytical science, regulatory scrutiny, and digital distribution are reshaping formulation choices, sourcing priorities, and market access strategies
The landscape surrounding androstenedione and its derivatives is undergoing a sequence of transformative shifts driven by scientific validation, regulatory recalibration, and distribution innovation. Advances in analytical chemistry and bioavailability research have sharpened understanding of derivative-specific pharmacokinetics, prompting formulators to re-evaluate excipient matrices and delivery strategies. Consequently, product development trajectories now prioritize derivative selection that aligns with targeted onset, duration, and safety profiles, and formulators increasingly integrate stability and impurity control earlier in the R&D lifecycle.At the same time, regulatory authorities across multiple jurisdictions are tightening scrutiny on manufacturing traceability and labeling accuracy, which has reoriented procurement toward suppliers with robust quality management systems and transparent supply chains. This regulatory emphasis has elevated certification and compliance credentials from nice-to-have attributes to decisive procurement criteria. As a result, sales and marketing teams must now translate technical compliance into accessible value propositions for both professional and consumer audiences.
Concurrently, digital distribution channels and direct-to-consumer models are reshaping accessibility and consumption patterns. Online platforms accelerate product discovery and comparative evaluation, but they also intensify reputational risk as regulatory nonconformances become more visible. This dynamic places a premium on proactive quality assurance and consumer education. Taken together, these shifts compel stakeholders to adopt integrated strategies that marry rigorous science with resilient supply chains and transparent go-to-market models.
Tariff-driven trade adjustments triggered supplier reshuffling, nearshoring, and intensified compliance coordination that reshaped procurement and operational priorities
The imposition of new United States tariff measures in 2025 introduced a consequential layer of commercial friction that rippled across procurement, pricing strategy, and supply chain resilience planning. Importers and manufacturers recalibrated supplier mixes in response to duty-related cost differentials, which in turn prompted reassessment of nearshoring, multi-sourcing, and inventory policies. Many organizations pivoted toward sourcing alternatives with lower tariff exposure or elevated domestic processing to mitigate landed cost increases and reduce exposure to future tariff volatility.In parallel, tariff-driven margin pressure stimulated operational efficiency initiatives across production and logistics functions. Organizations intensified cost-per-unit analysis and prioritized investments in process improvements that lower conversion costs and reduce waste. Procurement functions revised contract clauses to reflect tariff contingencies and to secure more favorable lead time terms. Importantly, compliance and customs teams became central to commercial decision-making, with cross-functional collaboration accelerating between regulatory, legal, and supply chain groups to ensure uninterrupted product availability.
Strategically, the tariff environment sharpened the focus on vertical integration opportunities and on developing closer relationships with compliant manufacturers and distributors. Companies with flexible manufacturing capacity and those able to demonstrate robust quality systems found new avenues to partner with foreign suppliers seeking compliant distribution channels in the U.S. market. These adaptations show how trade policy can catalyze structural change that extends beyond immediate cost impacts, influencing long-term supplier selection and operational architecture.
Segmentation-driven strategic differentials linking application-specific requirements, product chemistry, and distribution pathways to commercial and regulatory choices
Segmentation insights reveal where scientific properties, application needs, and commercial pathways intersect to create differential value and risk profiles. When viewed through application lenses such as cosmetics, dietary supplements (including bodybuilding, sports nutrition, and wellness subsegments), pharmaceuticals, and research, demand drivers and regulatory expectations diverge. Cosmetic formulators emphasize sensory compatibility and topical safety margins, while dietary supplement manufacturers prioritize bioavailability, dosing consistency, and claims substantiation for bodybuilding, sports nutrition, or general wellness products. Pharmaceutical and research applications demand the highest levels of impurity control, batch traceability, and documentation for reproducibility, which affects sourcing and manufacturing decisions.Product type segmentation into anhydrous and monohydrate forms further shapes formulation strategy, stability considerations, and storage logistics. Anhydrous variants often provide advantages in certain synthetic pathways and moisture-sensitive formulations, whereas monohydrate forms can affect solubility and hygroscopic behavior, influencing packaging and shelf-life management. Derivative-level differentiation across acetate, enanthate, propionate, and undecanoate drives pharmacokinetic selection for target use cases; each derivative presents distinct absorption and ester-cleavage profiles that guide clinical utility and consumer-facing positioning.
Distribution-channel segmentation shows divergent risk and opportunity dynamics between offline and online pathways. Offline distribution, comprising direct sales and distributor networks, often supports professional end users that require certificates of analysis and tailored technical support. Online distribution, spanning company websites and third-party e-commerce platforms, accelerates reach to retail consumers but heightens the need for transparent labeling and proactive customer education. End-user segmentation across clinics, contract research organizations, hospitals (private and public), and research institutions clarifies procurement protocols, volume requirements, and compliance expectations, thereby informing tailored commercial and regulatory engagement strategies.
Regional regulatory variability, manufacturing capacity, and logistical realities are prompting firms to recalibrate geographic footprints and compliance strategies
Regional dynamics remain a primary determinant of regulatory regimes, supply chain design, and commercial opportunity. In the Americas, regulatory frameworks and enforcement priorities vary across jurisdictions but generally emphasize traceability and product safety; supply chains here benefit from proximity to major end markets and advanced logistics infrastructure, supporting strategies that prioritize rapid replenishment and responsive commercial channels. Market participants active in the Americas often pursue partnerships with certified domestic manufacturers to mitigate trade friction and to align with local compliance expectations.Europe, Middle East & Africa present a mosaic of regulatory expectations and procurement models. European regulatory bodies place significant emphasis on impurity profiling, labeling accuracy, and post-market surveillance, prompting manufacturers to adopt elevated documentation and surveillance processes. Middle East procurement approaches differ by country, frequently reflecting public procurement processes and regional distribution hubs. Africa’s supply channels vary widely in maturity, leading suppliers to adapt packaging, cold chain logistics, and education initiatives to local infrastructure realities. Cross-region regulatory harmonization efforts and multilateral trade arrangements continue to influence supply route selection and compliance planning.
Asia-Pacific stands out for its deep manufacturing base and diverse regulatory regimes. Many participants in this region offer scale manufacturing capabilities, but buyers must assess supplier quality management systems and regulatory track records carefully. Regional innovation hubs are also advancing formulation science, which accelerates derivative-specific development and localized product differentiation. Given these contrasts across regions, firms are recalibrating their geographic footprints, balancing cost-efficient manufacturing with the need for predictable compliance and reliable logistics.
Competitive positioning hinges on quality systems, derivative specialization, strategic partnerships, and the ability to deliver compliant full‑service solutions
Competitive dynamics are shaped by a constellation of capability differentials that include quality management systems, regulatory expertise, manufacturing flexibility, and channel partnerships. Companies that demonstrate rigorous analytical controls, transparent documentation, and consistent supplier audits position themselves as preferred partners for pharmaceutical and research end users. Conversely, firms that excel at consumer-facing branding, educational outreach, and direct-to-consumer fulfillment find traction within cosmetics and dietary supplement channels, particularly where formulation and dosage clarity matter to end users.Several competitive vectors deserve attention: product portfolio breadth and derivative specialization, vertical integration of synthesis and finishing operations, and the agility to adapt packaging and labeling to meet diverse regulatory regimes. Market entrants often differentiate through niche derivative expertise or by offering high-touch technical support to contract research organizations and clinical partners. Established players leverage scale and distribution networks to secure long-term contracts with hospitals and large clinic groups, while nimble niche suppliers focus on rapid formulation iterations and targeted clinical collaborations.
Partnership strategies also influence competitive positioning. Strategic alliances with certified manufacturers, accredited testing laboratories, and logistics providers enable companies to offer turnkey solutions that reduce buyer procurement complexity. Intellectual property related to formulation and impurity control provides another axis of differentiation, particularly for firms seeking to move up the value chain from commodity supply toward differentiated therapeutic and performance-oriented products.
Prioritized tactical actions to align supplier controls, selective vertical integration, segment-focused go‑to‑market strategies, and resilient supply chain planning
Industry leaders should adopt a prioritized set of actions that align scientific rigor with commercial resilience and regulatory readiness. First, strengthen supplier qualification protocols and require comprehensive analytical documentation to reduce downstream compliance risk. This involves upgrading audit criteria, mandating robust certificates of analysis, and incorporating contingency clauses that address trade policy volatility. Second, invest in selective vertical integration or long-term co-manufacturing agreements to secure capacity for high-compliance segments such as pharmaceuticals and clinical research.Third, tailor go-to-market strategies by segment: develop clinically substantiated narratives and technical support for pharmaceutical and research clients, while creating consumer-friendly education campaigns for cosmetics and dietary supplement audiences that clearly communicate dosing, safety, and sourcing provenance. Fourth, enhance digital channel governance by instituting tighter product listing controls, proactive marketplace monitoring, and rapid takedown procedures to protect brand integrity and ensure regulatory compliance. Fifth, implement scenario-based supply chain planning that embeds tariff sensitivity, lead time variability, and nearshoring options into procurement models.
Taken together, these actions will help organizations reduce regulatory exposure, improve supply-chain predictability, and better align product offerings with the distinct expectations of clinics, hospitals, research institutions, and consumer-focused channels.
A cross‑disciplinary methodology combining primary stakeholder interviews, technical literature review, regulatory analysis, and supply chain mapping to ensure defensible strategic insights
The research methodology blends a cross-disciplinary approach that integrates primary stakeholder interviews, targeted technical literature review, regulatory source analysis, and supply chain evaluation to ensure robust and actionable findings. Primary inputs included structured discussions with formulation scientists, compliance officers, procurement leads, and clinical researchers to capture operational challenges and risk mitigation practices. Secondary inputs comprised peer-reviewed analytical studies, regulatory guidance documents, and publicly available quality standards to corroborate technical assertions and to validate analytical methods used in derivative characterization.Analytical rigor involved mapping the value chain from synthesis through distribution and end use, documenting where derivative chemistry interacts with formulation choices and regulatory checkpoints. Quality of evidence was assessed using predefined criteria that weighted direct, verifiable documentation more heavily than anecdotal observation. Cross-validation occurred by triangulating interview insights with documented regulatory notices and laboratory method references. Special attention was paid to trade policy changes and their documented effects on procurement decisions and logistics timelines.
Limitations are acknowledged; proprietary supplier data and confidential contractual terms were not accessible for all participants, and regional regulatory nuance can evolve rapidly. To mitigate these constraints, the approach prioritized sources with verifiable documentation and coupled them with expert interviews to interpret operational implications. The resulting methodology provides a defensible foundation for the strategic insights and recommendations presented herein.
Synthesis of strategic priorities emphasizing resilient suppliers, derivative‑aligned development, and governance measures to translate science into trusted commercial offerings
In closing, the androstenedione and derivative landscape is defined by an intersection of advancing scientific understanding, heightened regulatory expectations, and shifting commercial pathways. These dynamics create both risk and opportunity: organizations that proactively elevate quality assurance, adapt sourcing strategies in response to trade shifts, and tailor channels and messaging to segmented end users will secure competitive advantages. Conversely, firms that underinvest in compliance, supplier transparency, and digital governance risk supply disruptions and reputational exposure.Strategic priorities that emerge from this analysis include building resilient supplier networks, aligning product development with derivative-specific scientific evidence, and deploying targeted commercial strategies that resonate with distinct end-user needs. By operationalizing these priorities through upgraded audit regimes, selective vertical integration, and focused digital channel controls, stakeholders can reduce exposure to regulatory and trade shocks while positioning products for differentiated clinical or consumer value.
Overall, the path forward requires disciplined investment in quality systems, agile supply chain design, and clear communication strategies that translate technical strengths into trusted, market-ready offerings. Executives and functional leaders should use these conclusions to inform immediate tactical decisions and to shape medium-term capability building that will sustain competitive performance.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Androstenedione & Derivatives Market
Companies Mentioned
The key companies profiled in this Androstenedione & Derivatives market report include:- Bachem Holding AG
- BASF SE
- Cayman Chemical Company
- Evonik Industries AG
- Lonza Group AG
- Merck KGaA
- Steraloids, Inc.
- Thermo Fisher Scientific Inc.
- Wuxi AppTec Co., Ltd.
- Xi'an Gaoyuan Bio-Chem Co., Ltd.
- Zhejiang Xinan Chemical Industrial Group Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
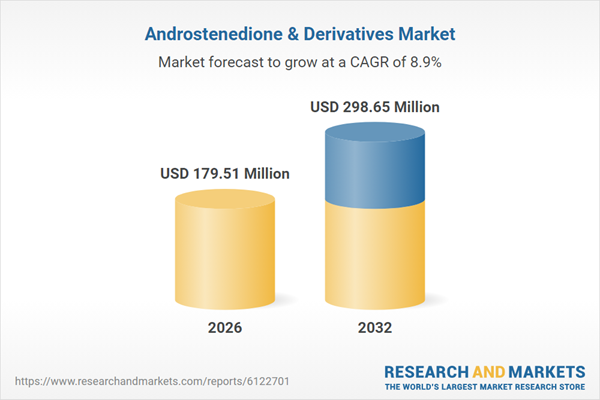
| Estimated Market Value ( USD | $ 179.51 Million |
| Forecasted Market Value ( USD | $ 298.65 Million |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |