Speak directly to the analyst to clarify any post sales queries you may have.
Framing thromboelastography as a pivotal diagnostic capability reshaping clinical workflows and influencing multidisciplinary patient management strategies
Thromboelastography has evolved from a specialized coagulation assessment to an integral component of perioperative decision making and critical care management. This introduction frames the diagnostic modality in the context of contemporary healthcare priorities, where rapid, comprehensive haemostasis assessment informs transfusion decisions, guides anticoagulant reversal, and supports individualized care pathways. Clinicians and laboratory leaders increasingly demand analytical tools that provide real-time, actionable information while integrating seamlessly into heterogeneous clinical environments.The technology’s capacity to profile clot formation, strength, and dissolution positions it at the intersection of surgical, trauma, and critical care pathways. Consequently, adoption drivers extend beyond test performance to encompass usability, turn‑around time, connectivity, and economic alignment with institutional purchasing models. This section outlines the foundational attributes of thromboelastography analyzers and situates them within a healthcare ecosystem that prioritizes adaptability, interoperability, and demonstrable impact on patient outcomes. By establishing this baseline, subsequent sections build upon the operational, regulatory, and commercial dynamics shaping procurement and clinical utilization.
Emerging technological integration, clinical practice realignment, and commercial model innovation that are redefining adoption and deployment of coagulation analyzers
The landscape for thromboelastography analyzers is experiencing transformative shifts driven by technological miniaturization, expanded clinical indications, and a reorientation toward point-of-care decision support. Advances in sensor design and consumable chemistry have shortened assay times and enhanced reproducibility, enabling deployment beyond central laboratories into operating rooms and intensive care units. This shift is reinforced by user-interface refinements and connectivity features that facilitate integration with electronic health records and clinical decision support systems, thereby improving the speed and traceability of interventions.Concurrently, clinical practice patterns are adapting to emphasize individualized hemostasis management across perioperative and trauma pathways. As a result, demand for flexible platforms that can transition between high-throughput reference settings and rapid point-of-care contexts is rising. Business model innovations have emerged in parallel, with procurement options such as leases, subscriptions, and hybrid financing aligning vendor incentives with long-term clinical outcomes. Regulatory and reimbursement environments are also evolving, encouraging evidence generation that demonstrates impact on transfusion rates, complication reduction, and length of stay. Together, these technological, clinical, and commercial shifts are reshaping how stakeholders evaluate and adopt thromboelastography systems.
Evolving trade dynamics and tariff implications driving supply chain resilience, procurement flexibility, and strategic sourcing for diagnostic equipment
Recent tariff policies in the United States have introduced layers of cost and logistical complexity into procurement channels for complex diagnostic equipment. Tariffs affect not only finished analyzers but also a global supply chain of critical consumables and spare parts, influencing vendor pricing strategies, inventory management, and regional distribution priorities. These measures have prompted manufacturers and distributors to reassess sourcing footprints, expedite localization of supply where feasible, and implement protective measures that preserve service continuity for clinical customers.Clinicians and hospital procurement teams face the operational implications of these trade shifts, which can include extended lead times for replacement components and recalibrated total cost of ownership calculations. To mitigate exposure, institutions are increasingly seeking contractual safeguards such as longer service agreements, priority stocking arrangements, and flexible procurement modalities that decouple capital expenditure from recurring consumable commitments. From a strategic perspective, the tariff environment is catalyzing greater emphasis on supply chain resilience, dual-sourcing strategies, and collaborative forecasting between clinical end users and vendors to maintain uninterrupted access to thromboelastography capabilities.
Deep segmentation analysis across end users, product families, assay technologies, clinical applications, and procurement models revealing tailored adoption drivers
Segmentation reveals nuanced adoption patterns and operational requirements across end users, product categories, technologies, applications, and purchasing modes. When viewed through the lens of end user distribution across hospitals, point-of-care settings, reference laboratories, and research facilities, distinct workflow imperatives emerge. Hospitals encompass both secondary and tertiary care institutions with differing patient acuity and surgical volumes that influence device throughput and service expectations. Point-of-care deployment within emergency departments and intensive care units emphasizes immediacy and ease of use, whereas reference labs, whether independent or national, prioritize throughput, standardization, and integration with laboratory information systems. Research laboratories, subdivided into academic and corporate environments, demand flexible protocols and modular platforms to support method development and investigational use.Product segmentation between microfluidic, non‑rotational, and rotational platforms further shapes procurement and clinical deployment strategies. Microfluidic solutions, available in reusable and single‑use formats, market an attractive balance of low sample volume and disposability for infection control, while non‑rotational platforms that present as benchtop or portable units cater to laboratory throughput or mobility needs. Rotational analyzers, spanning fully automated to semi‑automated designs, continue to serve settings that prioritize established performance metrics and robust quality control. Technology choices among kaolin‑activated, platelet mapping, and rapid assay variants reveal tradeoffs between comprehensive coagulation profiling and targeted, expedited assessments; kaolin activation subdivides into extrinsic and intrinsic activation pathways, platelet mapping discriminates between AA and ADP pathways, and rapid assays may be configured with heparinase or native protocols to address specific anticoagulant contexts.
Application segmentation-spanning coagulation monitoring, hemostasis management, and transfusion guidance-demonstrates how use cases inform platform selection and clinical pathways. Coagulation monitoring for critical care and surgical contexts demands fast, actionable readouts and straightforward interpretation, while hemostasis management during perioperative and preoperative phases often requires deeper integration with multidisciplinary protocols. Transfusion guidance in cardiac surgery and trauma settings places a premium on rapidity, reproducibility, and established decision thresholds. Finally, mode of purchase considerations-lease, purchase, or subscription-have subvariants that include finance versus operating leases, capital outlay versus operational expense treatments, and annual or monthly subscription cadences; these options materially affect budget planning, upgrade cycles, and vendor relationships, and they influence whether stakeholders prioritize total cost predictability, access to upgrades, or alignment to variable case volumes.
Comprehensive regional dynamics shaping clinical adoption, support infrastructure, and procurement approaches across disparate healthcare systems
Regional dynamics are shaping how thromboelastography capabilities are adopted, scaled, and supported across diverse healthcare systems. In the Americas, adoption is driven by a high prevalence of advanced surgical programs and trauma centers that prioritize real-time coagulation assessment, with established clinical pathways that facilitate integration into perioperative and critical care protocols. This region also emphasizes bundled procurement strategies and the maturation of point-of-care testing governance, which influences deployment patterns and training investments.Within Europe, Middle East & Africa, heterogeneous regulatory regimes and variable hospital resourcing create differentiated uptake trajectories. High‑resource centers in Western Europe pursue comprehensive integration and interoperability with digital health records, while emerging systems prioritize robust, cost‑effective platforms capable of operating with constrained infrastructure. The Middle East is expanding investments in tertiary care capabilities and specialized surgical services, which increases demand for advanced coagulation monitoring. Africa’s market dynamics often hinge on decentralized solutions and simplified workflows that support broader access in resource-limited settings.
Asia-Pacific exhibits a wide spectrum of adoption that ranges from rapid uptake in metropolitan centers with high surgical volumes to incremental introduction in markets focused on expanding trauma and cardiovascular services. Supply chain considerations, local regulatory pathways, and evolving reimbursement mechanisms shape how vendors approach market entry and expansion. Across all regions, support infrastructure for training, maintenance, and rapid consumable replenishment remains a decisive factor in sustained clinical utilization and long-term vendor relationships.
Competitive landscape dynamics driven by legacy manufacturers, agile innovators, and ecosystem partnerships shaping product and service differentiation
The competitive environment for thromboelastography analyzers is characterized by a mix of established diagnostics manufacturers, specialized start‑ups, and contract technology providers that together drive product evolution and market access strategies. Incumbent firms leverage legacy relationships with hospital systems and integrated service networks to offer end-to-end solutions including analyzer hardware, consumable supply chains, and long-term maintenance agreements. These players invest in regulatory pathways and clinical evidence generation to solidify adoption among conservative institutional buyers and to demonstrate improvements in patient management metrics.Emerging vendors emphasize portability, modular design, and software-enabled decision support to address unmet needs in point-of-care contexts. Their go‑to‑market strategies often prioritize partnerships with academic centers, clinical champions, and regional distributors to accelerate clinical validation and scale. Contract manufacturers and OEM partners contribute to supply chain flexibility by enabling rapid iteration of disposable components and microfluidic cartridges, while select software firms enhance device utility through analytics, cloud connectivity, and interoperability modules. Competitive advantage increasingly depends on a combined proposition of assay performance, workflow alignment, and commercial flexibility, as organizations evaluate vendors not only on technical merit but also on evidence of clinical benefit, service reliability, and total lifecycle support.
Actionable strategic priorities for manufacturers and providers to accelerate clinical adoption and fortify supply chains while aligning commercial models to healthcare needs
Industry leaders must orient strategies around clinical utility, supply chain robustness, and procurement flexibility to realize sustainable adoption of thromboelastography systems. Prioritizing integrations with electronic medical records and clinical decision pathways will accelerate clinician uptake by embedding results into established workflows and by reducing interpretive friction. Investing in evidence generation that links analyzer use to measurable reductions in transfusions, complications, and length of stay will strengthen reimbursement discussions and support broader institutional adoption. Manufacturers should collaborate with clinical champions to co‑develop training programs and decision algorithms that translate analytical outputs into standardized interventions across surgical and critical care teams.Operationally, diversifying manufacturing footprints and establishing regional stocking agreements can mitigate risk from trade disruptions and tariffs. Offering flexible purchasing models-such as subscription or hybrid arrangements that align with case volume variability-will appeal to hospitals balancing capital constraints and the need for predictable service. From a product perspective, developing modular platforms that can serve both high‑throughput reference labs and point‑of‑care environments reduces fragmentation and streamlines support. Finally, fostering partnerships with software and analytics providers to deliver post‑market evidence and remote performance monitoring will differentiate offerings and improve long‑term customer satisfaction.
Mixed methods research design combining stakeholder interviews, technical evaluation, and supply chain mapping to generate clinically actionable insights
The research methodology underpinning this analysis integrates a triangulated approach combining primary stakeholder input, device-level technical assessment, and supply chain mapping to ensure a practical and clinically grounded perspective. Primary engagement included structured interviews with clinicians, laboratory directors, procurement officers, and industry executives to capture decision criteria, operational constraints, and evidence needs. These qualitative insights were complemented by technical reviews of device architectures, assay chemistries, and connectivity frameworks to evaluate performance tradeoffs and integration challenges.Supply chain analysis involved mapping component sourcing, manufacturing footprints, and logistical channels to identify vulnerability points and mitigation strategies. Regulatory and reimbursement landscapes were reviewed to understand approval pathways and evidence requirements that impact adoption. Throughout the methodology, validation steps included cross‑referencing stakeholder feedback with device specifications and service models to reconcile user expectations with operational realities. This mixed methods approach provides a robust foundation for the conclusions and recommendations presented, ensuring they reflect both clinical realities and commercial imperatives.
Synthesis of technological, clinical, and commercial drivers that determine long-term adoption and clinical impact of coagulation analysis solutions
The conclusion synthesizes how technological advances, shifting clinical paradigms, and evolving procurement models are jointly reshaping the thromboelastography landscape. The technology’s increasing portability and connectivity, coupled with a broader set of clinical use cases, support expanded deployment across perioperative, trauma, and critical care environments. However, sustained adoption depends on demonstrable clinical value, resilient supply chains, and procurement arrangements that reflect institutional budgetary constraints and workflow needs.Stakeholders who align product development with clinical workflows, invest in evidence generation, and offer commercial flexibility will be best positioned to capture value and support improved patient outcomes. As healthcare systems continue to optimize for rapid, individualized care, thromboelastography analyzers that deliver reliable, timely insights and that integrate tightly into care pathways will be integral to modern coagulation management strategies.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Thromboelastography Analyzer Market
Companies Mentioned
The key companies profiled in this Thromboelastography Analyzer market report include:- Abbott Laboratories
- Becton Dickinson and Company
- Danaher Corporation
- Haemonetics Corporation
- HORIBA Ltd.
- Instrumentation Laboratory Company
- Medtronic plc
- Roche Holding AG
- Siemens Healthineers AG
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- Werfen Life Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
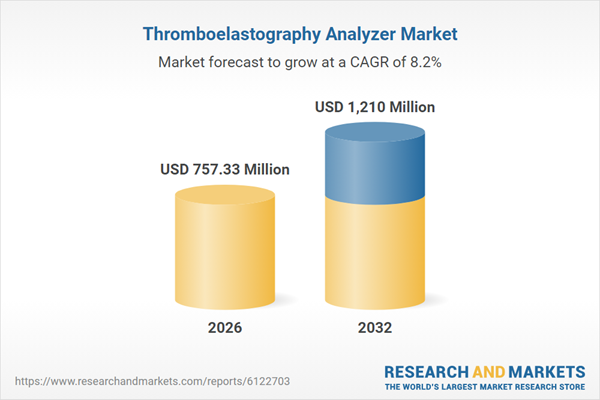
| Estimated Market Value ( USD | $ 757.33 Million |
| Forecasted Market Value ( USD | $ 1210 Million |
| Compound Annual Growth Rate | 8.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |