Speak directly to the analyst to clarify any post sales queries you may have.
Strategic overview of the follow-on infant formula landscape highlighting industry dynamics, consumer drivers, regulatory pressures, and innovation imperatives
The follow-on formula category occupies a unique intersection of nutrition science, caregiver behavior, and global trade dynamics. Recent advances in ingredient research, heightened regulatory scrutiny, and shifting consumer values have collectively elevated the strategic importance of this segment for manufacturers and retailers. Within this context, stakeholders must balance nutritional integrity, supply continuity, and commercial agility while navigating an environment of intensifying competition and regulatory complexity.This executive summary synthesizes the salient drivers shaping product development, channel performance, and operational resilience. It aims to provide a clear, concise orientation for executives, category managers, and policy teams who require a rapid yet substantive understanding of prevailing market mechanics and emergent risk vectors. The analysis foregrounds actionable themes such as formulation differentiation, packaging innovation, channel migration toward digital and hybrid commerce models, and the implications of trade policy shifts. By connecting these threads, the introduction sets the stage for targeted strategic responses that preserve consumer trust and optimize the route to market.
Emerging structural and behavioral shifts reshaping the follow-on formula category including formulation science, digital commerce adoption, and sustainability expectations
The follow-on formula landscape is undergoing a multi-dimensional transformation driven by scientific innovation, changing caregiver expectations, and evolving commercial infrastructure. On the product side, advances in ingredient science are enabling more sophisticated formulations that emphasize digestibility, targeted nutrient profiles, and the incorporation of functional components aligned to developmental milestones. These scientific gains are accompanied by stronger claims governance and heightened expectations from regulators and advocacy groups, which together are raising the bar for substantiation and labeling clarity.Simultaneously, consumer behavior is shifting; caregivers increasingly seek transparent sourcing, cleaner ingredient lists, and products that align with broader lifestyle choices, such as organic or plant-based alternatives. Digital commerce has accelerated discovery and direct-to-consumer fulfillment, compelling brands to invest in omnichannel strategies that integrate e-commerce, subscription models, and experiential education. Finally, sustainability has moved from a peripheral concern to a strategic imperative, catalyzing changes in packaging design, supply chain emissions accounting, and ingredient sourcing practices. These converging trends demand that organizations re-evaluate R&D priorities, marketing narratives, and operational capabilities to remain relevant and trusted in a rapidly evolving category.
Assessment of how the United States tariff adjustments in 2025 reverberate across supply chains, pricing mechanics, procurement strategies, and trade relationships
Adjustments to tariff policy in major trading jurisdictions can create ripple effects across procurement, inventory management, and retail pricing in the follow-on formula supply chain. When tariff changes are implemented, manufacturers experience a shift in input cost dynamics that can prompt reassessment of supplier contracts, distribution footprints, and hedging practices. Procurement teams typically respond by diversifying sourcing, seeking local or nearshored ingredient suppliers, or renegotiating terms to preserve margin and continuity of supply.Retailers and distributors also adapt commercial strategies; assortment planning may prioritize SKUs with more resilient cost structures, while promotional and pricing tactics are modified to reflect altered landed costs and consumer sensitivity. In parallel, logistics partners and contract manufacturers may accelerate efforts to optimize inbound freight and customs compliance processes to limit disruption. On the policy front, companies often deepen engagement with trade advisors and industry associations to monitor tariff implementation details and potential reciprocal measures. Across the board, increased transparency and scenario planning are essential to managing uncertainty, preserving consumer trust, and maintaining operational agility under a changing tariff regime.
Segment-driven intelligence revealing performance drivers across product forms, milk and product sources, pricing tiers, packaging formats, distribution routes, and developmental stages
Segment-level dynamics reveal differentiated opportunities and pressure points that inform product, channel, and go-to-market choices. Product form distinctions between liquid and powdered offerings shape manufacturing complexity, shelf life considerations, and logistics intensity; liquid formats often command convenience-oriented positioning and require distinct cold chain or sterile processing capabilities, whereas powdered variants emphasize portability, storage efficiency, and cost-to-serve dynamics. Milk source differentiation among cow milk, goat milk, and plant-based alternatives drives formulation pipelines and consumer targeting; cow milk remains a familiar baseline while goat milk and plant-based sources appeal to niche health or dietary preferences and necessitate tailored nutritional balancing.Product source segmentation into conventional and organic tiers influences supply chain traceability and certification burdens, and it also informs premiumization strategies and label claims management. Price tier classification across premium and standard positioning governs brand investment levels, promotional cadence, and distribution strategies, with premium lines often prioritizing ingredient provenance and certification as points of differentiation. Packaging type choices between pouch and tin affect shelf presence, perceptions of value and sustainability, and distribution efficiency. Distribution channel performance varies between convenience stores, online retail, pharmacies & drug stores, and supermarkets & hypermarkets, each offering distinct shopper journeys and promotional mechanics. Finally, developmental stage segmentation across Stage Five, Stage Four, Stage Three, and Stage Two guides ingredient profiles, nutrient density, and marketing messages tailored to caregiver expectations at different child growth phases.
Regional differentiation analysis illustrating demand patterns, regulatory contrasts, logistical considerations, and commercial tactics across global regions and trading blocs
Regional characteristics materially affect regulatory frameworks, consumer preferences, and supply chain design, creating distinct commercial playbooks for each geography. In the Americas, shopper familiarity with established dairy-based formulations coexists with growing interest in organic and convenience-led formats, while logistics networks and retail consolidation patterns influence assortment strategies and route-to-market economics. Across Europe, the Middle East & Africa, regulatory rigor around ingredient approvals and labeling claims is particularly salient, prompting companies to prioritize compliance, transparent communication, and localized product adaptations to meet diverse national requirements.The Asia-Pacific region exhibits rapid adoption of premium and imported products in many urban centers, coupled with high demand variability driven by cultural preferences and channel fragmentation. Regional supply chain resiliency is a common theme; manufacturers often maintain multi-jurisdictional sourcing strategies and regional manufacturing nodes to reduce lead times and manage tariff or regulatory volatility. Trade relationships, consumer trust in imported brands, and regional retail formats combine to create differentiated approaches to pricing, promotion, and portfolio localization across these broad geographies.
Competitive intelligence spotlighting strategic priorities of leading manufacturers, ingredient partners, private-label entrants, and route-to-market innovators in the follow-on formula sector
Competitive dynamics in the follow-on formula sector are shaped by a mix of large multinational manufacturers, specialized regional players, ingredient suppliers, and emerging private-label entrants. Leading manufacturers typically invest heavily in clinical research, ingredient partnerships, and quality assurance systems to underpin trust and support higher price tiers. Ingredient partners and contract manufacturers play an important role in enabling speed-to-market, particularly for novel formulations or plant-based blends, and strategic alliances can accelerate access to differentiated inputs while spreading compliance responsibilities.Private-label and value-oriented players exert pressure on pricing and drive efficiency improvements, prompting branded manufacturers to emphasize provenance, certification, and innovation to maintain differentiation. Channel specialists and digital-native brands have introduced direct-to-consumer models that bypass traditional distribution layers, creating opportunities for subscription models and richer consumer data capture. Across the competitive set, companies that integrate robust traceability, transparent communication, and nimble supply chain practices are better positioned to respond to regulatory changes and shifting consumer preferences while protecting brand equity and commercial margins.
Targeted playbook for industry leaders to accelerate growth through product optimization, supply resilience, channel evolution, regulatory alignment, and brand trust building
Industry leaders should prioritize a coordinated strategy that links product development, supply chain resilience, and consumer engagement. First, invest in formulation differentiation that balances scientifically validated benefits with clean-label expectations; this approach strengthens brand trust and supports premium positioning while meeting caregiver demand for transparent nutrition choices. Concurrently, deepen supplier relationships and diversify sourcing to mitigate disruption risks and enable swift shifts between origins when trade policy or logistics constraints arise.Channel strategies must evolve to reflect the increasing importance of e-commerce and hybrid fulfillment models. Brands should build capabilities for direct-to-consumer engagement, subscription services, and data-driven personalization, while maintaining strong partnerships with traditional retail to preserve shelf presence and impulse purchase opportunities. Operationally, firms should implement robust customs and tariff monitoring processes, scenario-sensitive inventory planning, and sustainable packaging initiatives to reduce environmental footprint and align with consumer values. Finally, enhance regulatory and quality assurance functions to accelerate compliance across jurisdictions and proactively communicate safety and provenance to caregivers, thereby protecting reputation and facilitating market access.
Transparent research approach combining primary stakeholder engagement, regulatory and supply chain analysis, and qualitative synthesis to ensure rigor and relevance
The analysis underpinning this executive summary draws on a mixed-methods research approach designed to ensure both breadth and depth of insight. Primary engagement included structured interviews with senior leaders across manufacturing, formulation science, retail, and distribution, together with consultations with regulatory experts and supply chain practitioners to capture practical responses to policy shifts and operational constraints. Secondary analysis comprised a comprehensive review of published regulatory guidance, trade policy notices, industry white papers, and company disclosures to triangulate themes and validate claims.Qualitative synthesis techniques were applied to reconcile differing stakeholder perspectives and to surface pragmatic implications for strategy and operations. Supply chain mapping and scenario analysis were used to explore resilience options and procurement trade-offs without producing probabilistic forecasts. Throughout, an emphasis was placed on transparency of assumptions, provenance of evidence, and the practical applicability of recommendations, ensuring that conclusions are grounded in observable industry behavior and documented regulatory developments.
Executive synthesis drawing together strategic implications for manufacturers, retailers, regulators, and investors operating within the evolving follow-on formula ecosystem
Taken together, the trends, segmentation dynamics, regional contrasts, and competitive moves described in this summary underscore a period of strategic rebalancing within the follow-on formula category. Manufacturers that prioritize scientifically credible differentiation, maintain flexible sourcing strategies, and invest in omnichannel capabilities will be better positioned to navigate regulatory complexity and shifting consumer expectations. Retailers and distributors that adapt assortment strategies and enhance collaboration with suppliers can retain resilience and preserve shopper trust amid changing cost structures.Policy shifts and trade adjustments act as accelerants to existing pressures rather than as isolated shocks, and companies that adopt proactive risk management, scenario planning, and clear consumer communication will minimize disruption. Ultimately, the combination of nutritional stewardship, operational agility, and authentic consumer engagement will determine which organizations capture long-term value and sustain trust in a category where safety, quality, and provenance are paramount.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Follow-on Formula Market
Companies Mentioned
The key companies profiled in this Follow-on Formula market report include:- Abbott Laboratories
- Arla Foods amba
- Ausnutria Dairy Corporation Ltd.
- Beingmate Baby & Child Food Co., Ltd.
- Danone S.A.
- Feihe International Inc.
- Fonterra Co-operative Group Limited
- Guangdong Beingmate Baby & Child Food Co., Ltd.
- Meiji Holdings Co., Ltd.
- Nestlé S.A.
- Reckitt Benckiser Group plc
- Royal FrieslandCampina N.V.
- Synutra International, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
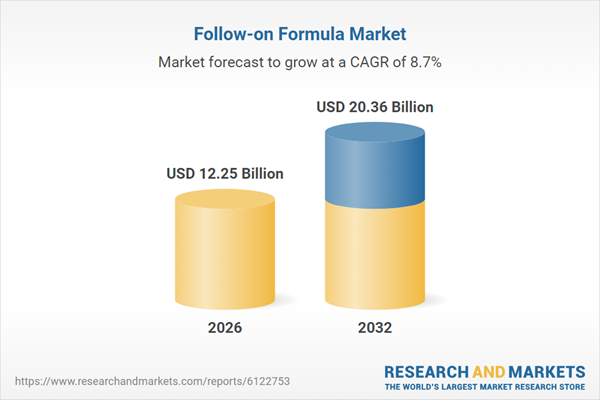
| Estimated Market Value ( USD | $ 12.25 Billion |
| Forecasted Market Value ( USD | $ 20.36 Billion |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |