Speak directly to the analyst to clarify any post sales queries you may have.
Hip surgery instrument sets have become a strategic lever for outcomes, OR efficiency, and supply resilience across sites of care
Hip surgery instrument sets sit at the intersection of clinical precision and operational discipline. As hip arthroplasty volumes remain structurally supported by aging populations and higher expectations for mobility, hospitals and ambulatory surgery centers are under persistent pressure to deliver consistent outcomes with shorter lengths of stay and tighter cost controls. In that environment, instrument sets are not merely accessories to implants; they determine how predictably a team can execute bone preparation, implant positioning, and closure while meeting sterilization standards and turnover targets.At the same time, the instrument landscape is expanding. Beyond traditional reusable stainless-steel trays, facilities now evaluate single-use components, hybrid configurations, and vendor-managed logistics to mitigate contamination risk and manage reprocessing constraints. Clinical teams increasingly ask for compatibility across implant platforms and for ergonomics that reduce fatigue during longer cases. Sterile processing departments, facing staffing gaps and capacity limits, prioritize simplified tray architecture, reduced instrument counts, and clear labeling to avoid delays and errors.
Consequently, the executive conversation has shifted from “which set do we need” to “which system best supports our model of care.” The answer depends on procedure mix, site of service, infection prevention requirements, surgeon preference governance, and procurement risk tolerance. This summary frames the most important forces shaping demand and adoption, clarifies how tariff dynamics can alter sourcing decisions, and highlights how segmentation and regional patterns influence competitive strategy.
Standardization, outpatient pathways, digital-enabled workflows, and infection prevention are redefining what modern hip instrument sets must deliver
The hip surgery instrument set landscape is being reshaped by a convergence of clinical standardization, operational constraints, and technology-led differentiation. One transformative shift is the rising emphasis on procedural consistency across multi-site health systems. Value-based care incentives and internal quality dashboards are encouraging service-line leaders to reduce variation in instruments and workflows. This has accelerated interest in standardized tray configurations, color-coded organization, and instrument rationalization programs that preserve surgeon-critical tools while eliminating redundancy that slows counting, cleaning, and assembly.In parallel, the site-of-service migration continues to influence instrument design priorities. As outpatient and short-stay pathways expand for appropriate candidates, instrument sets must support rapid room turnover and predictable setup, especially in ambulatory environments where sterile processing capacity may be limited. This has elevated demand for lighter trays, fewer layers, simplified reprocessing instructions, and packaging designed for quick verification. Facilities are increasingly sensitive to the hidden time costs of complex sets-missing instruments, ambiguous labeling, and inconsistent layouts-which can cascade into schedule disruptions.
Another major shift is the growing interplay between instrumentation and digital planning. While navigation and robotic assistance are often discussed as implant-adjacent technologies, they also place new requirements on instrument kits: calibration tools, tracking arrays, compatible reamers and broaches, and validated workflows for equipment checks. Even when advanced enabling technology is not used, preoperative templating and imaging-led planning have raised expectations for instrument availability and compatibility, particularly for complex anatomies and revision cases.
Infection prevention and regulatory scrutiny are also reshaping what “good” looks like. Increased attention to instrument cleanliness verification, traceability, and documentation has pushed manufacturers and providers toward designs that reduce lumens, minimize hard-to-clean features, and support validated cleaning processes. This is reinforced by heightened awareness of biofilm risk and the operational reality that reprocessing errors are more likely when trays are overstuffed or instruments are difficult to disassemble.
Finally, supply chain resilience has moved from a procurement slogan to a measurable operational requirement. Health systems are increasingly evaluating dual sourcing, local or regional manufacturing footprints, and service-level agreements that cover consignment replenishment, emergency loaners, and turnaround commitments. In this context, differentiation increasingly comes from the total system-training, instrumentation support, field service, and sterilization compatibility-rather than from instrument metallurgy alone. These shifts collectively raise the bar for vendors and providers, making instrumentation strategy a critical input to both clinical performance and operational capacity.
Tariff-driven cost volatility in 2025 is reshaping sourcing, contracts, and design choices for hip instrument sets without slowing clinical need
United States tariff dynamics in 2025 are expected to influence the hip surgery instrument set ecosystem through cost structure, sourcing strategies, and contractual behavior rather than through abrupt changes in clinical demand. Instrument sets rely on globally distributed supply chains for stainless steel inputs, precision machining, surface finishing, packaging materials, and in some cases subassemblies that are integrated into final trays. When tariffs raise the landed cost of components or finished instruments, suppliers often respond by repricing selected SKUs, adjusting discount structures, or rebalancing which products are fulfilled from which facilities.The immediate operational impact tends to be felt in procurement cycles. Health systems negotiating new capital and service agreements may encounter shorter price-hold windows and more frequent surcharge language tied to trade policy changes. This can complicate long-term standardization plans, particularly when facilities are consolidating vendors and attempting to lock in predictable total cost of ownership. In response, many buyers are strengthening their contract governance, requiring clearer transparency on pass-through mechanisms and demanding contingency planning for backorders.
Tariff pressure can also accelerate a shift toward design and packaging optimization. Manufacturers facing margin compression often pursue tray rationalization, modularity, and commonality across systems to reduce unit costs and simplify inventory. Over time, these engineering and operations changes can benefit providers through more streamlined sets, but the transition period may introduce SKU changes, substitute components, or revised cleaning instructions that require staff retraining.
Another important effect is the renewed evaluation of domestic or nearshore production for certain instrument families. While moving manufacturing is not trivial in a regulated environment-requiring process validation, supplier qualification, and consistent quality systems-tariffs can tilt the economic calculus toward investing in local capacity for high-volume instruments. Even without full relocation, suppliers may increase final assembly, finishing, or packaging domestically to reduce exposure.
On the provider side, tariffs reinforce the need to differentiate between price and availability risk. A lower-cost source that is more exposed to policy volatility may create downstream costs through expedited shipping, case delays, or higher safety-stock requirements. Conversely, premium suppliers may justify pricing through stronger continuity programs, local inventory buffers, and rapid loaner response. In 2025, the most capable organizations are likely to treat tariff-driven volatility as a catalyst to mature their instrumentation strategy-aligning clinical standardization with sourcing resilience, building cross-functional playbooks, and ensuring that sterile processing and OR leadership are part of contract decisions rather than downstream recipients of supply chain surprises.
Segmentation shows instrument-set preferences diverge sharply by procedure type, product strategy, care setting realities, and channel economics
Segmentation patterns reveal that purchasing behavior in hip surgery instrument sets is strongly shaped by where the set is used, how it is used, and the clinical complexity it must support across product type, application, end user, and distribution channel. Demand for total hip replacement instrumentation continues to prioritize comprehensive broaching, reaming, and trialing workflows with a strong focus on repeatability and speed, while partial hip replacement sets emphasize targeted femoral preparation and often compete on simplicity, tray weight, and faster turnover for fracture pathways. Meanwhile, hip revision surgery instrumentation is defined by variability and contingency planning; it typically requires expanded extraction tools, modular stems support, and adaptable reaming options, which elevates the value of robust loaner programs and rapid availability.From a product standpoint, the contrast between reusable and single-use instruments is becoming more nuanced. Reusable sets remain the backbone of most programs due to familiarity, durability, and per-case economics when reprocessing capacity is stable. However, single-use instruments are gaining consideration in contexts where sterile processing bottlenecks create schedule risk or where facilities seek to reduce cleaning complexity for specific tools. In practice, hybrid strategies are increasingly common, with reusable trays supplemented by single-use items in pain points such as cutting guides, suction tips, or specialty drivers, depending on facility policy and surgeon preference.
End-user dynamics further sharpen segmentation. Hospitals tend to manage broader case complexity, including revisions and comorbid patients, which drives demand for complete systems, deeper inventory, and service support that can handle peaks in volume. Ambulatory surgery centers, by contrast, often prioritize lean sets, predictable scheduling, and minimized reprocessing burden, making tray simplification and fast setup central to value. Orthopedic specialty clinics that perform procedures or support follow-up care influence instrument decisions through surgeon preference alignment and standardized protocols, often serving as hubs for evaluating new tray configurations and workflow updates.
Distribution channel differences also shape adoption. Direct sales relationships frequently matter when systems are complex and require on-site support, training, and responsive troubleshooting. Distributors and third-party logistics models can be advantageous for reaching smaller facilities or expanding geographic coverage, but they heighten the importance of consistent service levels, instrument tracking, and clear accountability for loaners and maintenance. Across segments, the most successful offerings align instrumentation design with the realities of reprocessing, staffing, and scheduling, rather than treating the tray as a static list of tools. This segmentation view underscores a central takeaway: the best-fit instrument strategy is rarely universal, and competitive advantage increasingly comes from tailoring kits and service models to the operational profile of each care setting.
Regional realities across the Americas, Europe, Middle East & Africa, and Asia-Pacific reshape instrument requirements and go-to-market execution
Regional dynamics demonstrate that adoption and purchasing priorities for hip surgery instrument sets are influenced by reimbursement structures, regulatory expectations, infrastructure maturity, and the pace of outpatient migration across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, large integrated delivery networks often lead with standardization programs and vendor consolidation, using governance models to reduce tray variation while demanding strong local inventory and responsive field support. The growing footprint of outpatient joint replacement also increases interest in lighter sets and streamlined workflows, particularly where facility networks include both hospitals and ambulatory sites that must share assets.In Europe, procurement is frequently shaped by centralized tendering and strong emphasis on compliance, documentation, and validated reprocessing. Instrumentation suppliers often compete on traceability, cleaning compatibility, and lifecycle support, alongside ergonomic design and clinical performance. The region’s diverse healthcare systems create a mosaic of preferences, but the common thread is rigorous scrutiny of total cost and sustainability considerations, which can influence choices around reusable tray longevity and packaging waste.
Across the Middle East & Africa, investment in surgical capacity and hospital infrastructure can drive demand for complete instrument systems and training-intensive partnerships. Facilities that are scaling orthopedic programs prioritize dependable availability, robust education, and service models that can support skill development and consistent technique. At the same time, variability in logistics and local sterilization capacity can push buyers toward solutions that reduce complexity and enhance reliability under constrained conditions.
In Asia-Pacific, a combination of expanding access to orthopedic care, rapid hospital development, and increasing procedural volumes is shaping diverse instrument needs. Major metropolitan centers may adopt advanced workflows, including enabling technologies that require specialized accessories and calibration tools, while other settings focus on durable, cost-effective trays that can withstand high utilization. Supply chain resiliency and lead-time management are especially salient in geographically dispersed markets, making local stocking strategies and responsive maintenance support key differentiators.
Taken together, regional insights reinforce that “one global tray” rarely optimizes outcomes and operations everywhere. Successful strategies adapt instrument configurations, service commitments, and training models to the realities of each region’s care pathways, regulatory environment, and infrastructure constraints, while maintaining consistent quality standards that protect patients and preserve clinical confidence.
Company differentiation increasingly hinges on operational support, tray rationalization, lifecycle service, and interoperability beyond the instruments themselves
Competition in hip surgery instrument sets is defined by a mix of orthopedic incumbents, specialized instrumentation manufacturers, and service-oriented partners that differentiate through reliability, compatibility, and operational support. Leading companies tend to emphasize design precision, durable materials, and cohesive ecosystems that pair instruments with implant systems and procedural workflows. Increasingly, they also compete on what happens outside the tray: education, on-site support, preventive maintenance, and the ability to provide loaners or backup sets without disrupting schedules.A central theme in company positioning is instrument rationalization without sacrificing surgeon confidence. Firms that can demonstrate reduced tray counts, simplified layouts, and validated cleaning pathways often win in environments where sterile processing is constrained. At the same time, suppliers serving high-complexity centers invest in modular expansion sets and revision-ready tools that can be rapidly deployed when intraoperative findings change. This ability to support both routine and complex cases-without forcing hospitals to overstock-is becoming a key differentiator.
Another area of rivalry is interoperability and lifecycle management. Buyers increasingly demand clearer policies on instrument refurbishment, replacement cycles, and compatibility across generations of implants. Companies that provide traceability features, maintenance documentation, and consistent availability of spare parts reduce operational friction and build long-term trust. In addition, as digital surgery ecosystems expand, firms that can integrate instrumentation with enabling technology requirements and provide structured training pathways are better positioned to defend accounts and expand within service lines.
Finally, service consistency is becoming a deciding factor in competitive evaluations. Facilities expect predictable turnaround on loaners, rapid resolution of missing or damaged instruments, and proactive communication when changes occur in SKU design or cleaning instructions. Companies that treat instrumentation as a managed service-aligning field teams, logistics, and sterile processing education-are increasingly favored over vendors that focus solely on the physical kit. This competitive landscape rewards those who translate engineering excellence into measurable operational reliability for OR teams and sterile processing departments alike.
Leaders can win by standardizing governance, optimizing trays, building tariff-resilient sourcing plans, and managing instruments as a lifecycle system
Industry leaders can strengthen performance and resilience by treating hip surgery instrument sets as a clinical-operational platform rather than a static procurement item. Start by aligning surgeon leadership, sterile processing, infection prevention, and supply chain teams around a shared definition of success that includes setup time, missing-instrument rates, cleaning complexity, and intraoperative flexibility. This alignment enables governance that respects surgeon technique while reducing avoidable variation across sites.Next, prioritize tray optimization as a measurable program. Conduct instrument utilization reviews to remove rarely used items, reconfigure layouts for faster counting, and standardize labeling to reduce assembly errors. Where outpatient pathways are expanding, evaluate lighter configurations and consider hybrid approaches that use single-use items strategically to relieve reprocessing bottlenecks without sacrificing consistency. Ensure any change includes training, updated instructions for use, and competency checks in sterile processing to avoid unintended disruption.
Given tariff and supply volatility, strengthen sourcing resilience through scenario planning. Negotiate contracts that define price-adjustment mechanisms clearly, include service-level commitments for loaners and emergency fulfillment, and establish escalation paths for shortages. Where feasible, diversify critical instrument sources or maintain contingency sets for high-impact procedures such as revisions. Integrate these plans with scheduling leadership so that clinical operations are not surprised by supply constraints.
Finally, invest in lifecycle management. Define refurbishment intervals, inspection standards, and replacement triggers for high-wear tools such as reamers, broaches, and cutting instruments. Use tracking and traceability capabilities to connect instrument condition to outcomes like case delays or reprocessing rework. Over time, this turns instrumentation into a continuously improved system that supports predictable performance, reduces avoidable downtime, and sustains surgeon confidence across expanding sites of care.
A triangulated methodology blends stakeholder interviews, regulatory and technical review, and segmentation-led validation to ensure decision-ready insights
The research methodology for this report combines structured primary engagement with rigorous secondary review to provide a practical, decision-oriented view of hip surgery instrument sets. Primary inputs include interviews and discussions with stakeholders across the care continuum, such as orthopedic surgeons, operating room leaders, sterile processing professionals, supply chain executives, and distribution specialists. These conversations focus on real-world workflows, pain points in tray management, adoption criteria for reusable and single-use strategies, and expectations for service support and training.Secondary research synthesizes publicly available regulatory guidance, standards related to reprocessing and device quality systems, corporate filings and product documentation, tender and procurement practices where accessible, and technical literature relevant to orthopedic instrumentation design and sterilization compatibility. This step helps validate terminology, map product and service characteristics, and identify patterns in purchasing behavior across care settings.
To ensure analytical consistency, findings are triangulated across sources and organized using a structured segmentation framework covering product type, application, end user, and distribution channel, then interpreted through a regional lens. The approach emphasizes internal validity through cross-checking claims, resolving discrepancies via follow-up review, and prioritizing evidence that reflects current operational realities. The result is a grounded narrative that supports executive decision-making around standardization, supplier selection, and operational readiness without relying on speculative assumptions.
Instrument sets are now a cornerstone of scalable hip care, linking clinical consistency, sterile processing capacity, and resilient supply strategy
Hip surgery instrument sets are increasingly central to how organizations deliver consistent orthopedic care at scale. What once appeared to be a technical purchasing decision now influences OR efficiency, sterile processing performance, infection prevention confidence, and the ability to expand outpatient pathways without compromising quality. As providers face staffing constraints and higher expectations for documented reprocessing, the design and manageability of trays matter as much as the instruments’ mechanical function.Looking across the landscape, the direction is clear: fewer, smarter, and more standardized sets supported by stronger service models. Providers are rewarding suppliers that can simplify complexity, ensure availability, and demonstrate compatibility with evolving workflows, including digitally enabled planning and, where relevant, advanced surgical systems. Meanwhile, trade and tariff pressures underscore that resilience must be built into contracts and sourcing strategies rather than treated as an afterthought.
Ultimately, the organizations that perform best will be those that connect clinical priorities with operational reality. By treating instrumentation as a lifecycle-managed platform-continuously optimized, monitored, and supported-leaders can protect patient outcomes while improving throughput and reducing avoidable friction across the perioperative pathway.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Hip Surgery Instrument Set Market
Companies Mentioned
The key companies profiled in this Hip Surgery Instrument Set market report include:- Acumed LLC
- Aesculap, Inc.
- Arthrex, Inc.
- B. Braun Melsungen AG
- Conmed Corporation
- DJO, LLC
- Exactech, Inc.
- HipInnovation LLC
- Integra LifeSciences Corporation
- Medacta International SA
- Medtronic plc
- MicroPort Orthopedics, Inc.
- MicroPort Scientific Corporation
- Orthofix Medical Inc.
- Smith & Nephew plc
- Stryker Corporation
- Trauson Holdings Co., Ltd.
- Wright Medical Group N.V.
- Zimmer Biomet Holdings, Inc.
- Zimmer, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
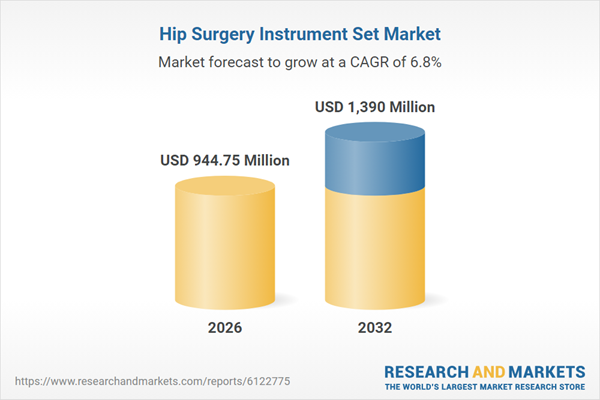
| Estimated Market Value ( USD | $ 944.75 Million |
| Forecasted Market Value ( USD | $ 1390 Million |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |