Speak directly to the analyst to clarify any post sales queries you may have.
Endotoxin testing systems are becoming strategic release enablers, reshaping quality control priorities across pharma, biotech, and medical device manufacturing
Medical endotoxin testing systems sit at the center of patient safety, product quality, and manufacturing continuity. Whether a therapy is an injectable small molecule, a complex biologic, a cell-based product, or a device that contacts blood, controlling pyrogenic risk is non-negotiable. The practical reality is that endotoxin testing is not simply a quality-control checkbox; it is a release gate that can accelerate or stall production, shape inventory strategy, and define an organization’s risk posture.In recent years, the sector has been reshaped by the convergence of more sensitive products, tighter turnaround expectations, and heightened scrutiny of data integrity and method suitability. At the same time, labs are expected to deliver results with fewer skilled analysts, while supply-chain volatility continues to pressure reagent sourcing and instrument availability. Consequently, leaders are rethinking the balance among traditional methods, alternative assays, automation, and integrated digital workflows.
This executive summary distills the pivotal forces influencing adoption, investment, and competitive differentiation in medical endotoxin testing systems. It frames where operational bottlenecks tend to emerge, how regulatory and sustainability priorities are influencing method choices, and which segments are demonstrating the strongest appetite for modernization. The goal is to provide decision-makers with a structured lens for evaluating near-term actions that reduce release friction while strengthening compliance and resilience.
Automation, recombinant alternatives, digital compliance, and multi-site standardization are redefining endotoxin testing from a lab task into an enterprise capability
The landscape is undergoing a structural transition from manual, operator-dependent workflows toward more standardized, automation-ready testing ecosystems. While gel-clot and kinetic LAL methods remain widely entrenched due to familiarity and established procedures, many organizations are now prioritizing repeatability, audit-readiness, and throughput over legacy comfort. This shift is particularly visible in high-volume environments where time-to-result and investigation avoidance directly influence supply availability.A second transformative shift is the accelerating evaluation and adoption of non-animal derived alternatives, particularly recombinant approaches. This movement is driven by a combination of sustainability objectives, long-term supply security concerns, and method performance considerations. As more regulators clarify expectations around validation, comparability, and method suitability, organizations are increasingly willing to run structured bridging studies and rework specifications to enable broader deployment beyond pilot programs.
In parallel, digitalization is changing the definition of a “testing system.” Instruments, readers, and incubators are increasingly expected to integrate with laboratory information management systems, enforce role-based access, support electronic records, and generate audit trails that withstand inspection scrutiny. The emphasis on data integrity is also pushing laboratories to minimize manual transcription and to standardize templates, calculations, and exception handling.
Finally, the sector is adapting to a more distributed manufacturing reality. With the growth of multi-site networks, contract manufacturing, and regionalized supply strategies, endotoxin testing must be transferable and consistent across laboratories with varying maturity levels. This is prompting greater interest in harmonized methods, centralized training models, vendor-supported qualification packages, and pre-configured workflows that reduce variability without sacrificing scientific rigor.
United States tariffs in 2025 are reshaping endotoxin testing economics, pushing buyers toward resilient sourcing, standardization, and total-cost-driven method choices
The introduction and expansion of United States tariffs in 2025 creates tangible operational consequences for endotoxin testing systems because the category depends on a globalized supply chain of components, consumables, and specialized materials. Many instruments incorporate electronics, optical subsystems, heaters, and precision plastics that can be exposed to tariff schedules depending on country of origin and classification. Even when final assembly occurs domestically, upstream subcomponents can elevate landed cost and complicate lead-time management.Reagents and consumables may face a different set of tariff sensitivities, but the impact can be just as disruptive. Endotoxin testing depends on consistent lot performance, controlled storage, and reliable replenishment. When tariffs raise the cost of imported cartridges, microplates, tips, water systems components, or critical reagents, procurement teams often respond by consolidating suppliers, negotiating longer-term agreements, or increasing safety stock. However, these mitigations can increase working capital requirements and introduce new expiry-management burdens.
Tariff pressure also influences technology decisions. Organizations weighing capital purchases may accelerate instrument standardization to reduce the variety of spare parts and service contracts across sites. Conversely, some may defer upgrades, extracting more life from existing readers and incubators to avoid near-term capital spikes. In regulated settings, deferral carries its own risks if older systems lack adequate data integrity features or if serviceability becomes uncertain due to parts availability.
Over time, the most enduring effect may be the renewed emphasis on supply-chain resilience and total cost of ownership. As tariffs amplify price variability, quality organizations are likely to place greater value on vendors that can demonstrate multi-region manufacturing, clear traceability, stable lead times, and strong technical support. Companies that proactively model tariff exposure, qualify alternate sources, and embed flexibility into method selection will be better positioned to avoid release delays and unplanned deviations.
Segmentation insights show distinct buying logic across assay types, sample matrices, end users, and workflow automation as labs pursue faster, defensible release decisions
Key segmentation patterns reveal a market where needs diverge sharply based on method preference, workflow maturity, and the regulated context of the end product. By product type, demand spans instruments and readers that anchor kinetic and endpoint measurements, reagents and kits that determine assay performance, accessories and consumables that stabilize routine execution, and software that increasingly governs data integrity and traceability. Buyers are no longer evaluating these components independently; they are seeking validated, interoperable stacks that reduce variability from sampling through reporting.By test type, gel-clot remains important where simplicity and low equipment overhead matter, yet it faces pressure in environments that require richer quantitative information and higher throughput. Kinetic turbidimetric and kinetic chromogenic approaches continue to anchor many QC programs because they align with established practices and compendial familiarity, but they are being re-implemented with stronger controls, improved automation, and tighter integration into electronic workflows. Alongside these, recombinant factor-based methods are gaining traction as organizations pursue animal-free alternatives, aiming to strengthen supply security and align with sustainability commitments while maintaining method suitability across diverse matrices.
By sample type, injectable drug products and raw materials demand stringent control and robust interference handling, particularly for complex formulations and high-protein matrices. Medical devices introduce a distinct set of extraction and recovery considerations that drive method selection and validation depth. Water systems, including purified water and water-for-injection distribution points, sustain continuous monitoring needs where rapid turnaround and trending can prevent broader contamination events. Biological products, including advanced modalities, increase the importance of matrix-specific validation and contamination control across upstream and downstream processing.
By end user, pharmaceutical and biotechnology manufacturers typically prioritize batch-release efficiency, global method harmonization, and inspection readiness. Contract development and manufacturing organizations emphasize flexibility, multi-client compliance, and rapid method transfer across platforms. Hospital and clinical laboratories, where applicable, tend to focus on operational simplicity and reliability, often balancing testing rigor with resource constraints. Academic and research institutions, while not always bound by commercial release pressures, can be influential early adopters when exploring alternative methods and novel workflows.
By application, the strongest pull is in product release testing, where delays translate directly into supply risk. In-process monitoring is increasingly valued for earlier detection and reduced deviation cost, especially in complex biologic manufacturing. Sterility assurance programs intersect with endotoxin control through environmental and process discipline, reinforcing demand for integrated quality systems rather than standalone assays. Quality control and quality assurance oversight further shape purchasing priorities by demanding audit trails, consistent training, and defensible investigation pathways.
By technology and workflow orientation, laboratories are choosing among traditional manual assays, semi-automated systems that reduce hands-on time, and fully automated platforms that standardize pipetting, incubation, reading, and calculations. This is complemented by a growing focus on rapid testing and at-line strategies in facilities seeking shorter release cycles. Across all segments, the decisive differentiator is no longer the assay alone, but the operational system surrounding it-validation support, digital integration, reagent continuity, and the ability to manage interference across real-world samples.
Regional insights highlight how the Americas, EMEA, and Asia-Pacific differ in compliance focus, sustainability adoption, and automation readiness for endotoxin programs
Regional dynamics reflect differences in regulatory emphasis, manufacturing footprints, and modernization pace. In the Americas, strong biologics and device manufacturing activity sustains continuous demand for robust, inspection-ready endotoxin control. Organizations in the United States and Canada are particularly focused on data integrity, method suitability documentation, and harmonized practices across multi-site networks, which supports investment in integrated software, standardized platforms, and vendor-led validation packages.Across Europe, the Middle East, and Africa, priorities frequently converge around harmonization and sustainability, with heightened interest in reducing reliance on animal-derived reagents and improving supply stability. Mature manufacturing hubs in Western Europe are leaning into digital workflows and standardized qualification approaches, while emerging production centers within the broader region often prioritize scalable systems that can be upgraded from manual to semi-automated operation as volume and regulatory expectations rise.
In Asia-Pacific, the combination of expanding pharmaceutical production, growing biologics capacity, and increasing medical device exports is accelerating adoption of modern endotoxin testing infrastructure. Many organizations are building new facilities or upgrading laboratories to meet global client expectations, which can shorten the replacement cycle for instruments and increase openness to automation. At the same time, procurement decisions in the region can be highly sensitive to lead times, local service coverage, and the availability of training resources, elevating the importance of vendor presence and distributor capability.
Across regions, the common theme is a move toward operational resilience. As supply chains and regulatory expectations evolve, laboratories are converging on solutions that can be consistently executed, transferred between sites, and defended under inspection. Regional nuances influence the speed and sequence of adoption, but the underlying direction is the same: endotoxin testing is being modernized as a foundational quality capability rather than maintained as a static legacy procedure.
Company dynamics increasingly reward integrated platforms, recombinant method leadership, and audit-ready service models that reduce variability and protect batch release timelines
Competition centers on the ability to deliver reliable assay performance, consistent reagent supply, and inspection-resilient workflows. Established providers maintain strong positions where long-standing LAL practices dominate, leveraging broad reagent portfolios, reader ecosystems, and service networks. However, differentiation is increasingly tied to how well companies help customers reduce variability through standardized protocols, matrix interference troubleshooting, and end-to-end documentation.A notable theme is the intensifying push toward integrated solutions. Vendors that combine instruments, validated software, and consumables with clear qualification guidance can reduce buyer burden, especially for organizations managing multiple sites or multiple product types. This integration is particularly valuable for contract manufacturers that need repeatable execution across diverse client methods and for large pharma that must enforce consistent practices across global networks.
Another differentiator is leadership in recombinant and other alternative approaches. Companies investing in robust validation support, comparability data packages, and regulatory-ready documentation are gaining consideration from organizations that previously postponed method migration. In parallel, suppliers are improving ease of use through simplified workflows, enhanced robustness to common interferences, and clearer guidance on sample preparation.
Service and supply continuity increasingly shape vendor selection. Buyers are evaluating not only assay specifications but also lot-to-lot consistency, shelf-life management, cold-chain reliability, and regional availability. Training quality, responsiveness during investigations, and the ability to support audits can be decisive factors, particularly when a deviation threatens batch release. As a result, vendors that operate as partners in quality outcomes-rather than commodity suppliers-are more likely to be embedded in long-term standardization plans.
Leaders can reduce batch-release friction by modernizing workflows, validating resilient methods, aligning procurement with quality, and strengthening digital governance
Industry leaders can strengthen endotoxin control by treating testing as a release-critical value stream rather than an isolated QC step. A practical first move is to map the end-to-end workflow-from sampling and sample preparation through incubation, reading, calculation, and review-to identify where delays and variability originate. This enables targeted investments such as automating repeatable steps, tightening incubation control, or standardizing calculation templates to reduce investigation risk.Method strategy should be revisited with a dual focus on performance and resilience. Organizations can run structured evaluations that compare traditional LAL methods with recombinant options using representative matrices and realistic operating conditions. When recombinant methods are pursued, bridging plans should include predefined acceptance criteria, robust interference evaluation, and clear documentation pathways that support future inspections and method transfer.
Procurement and supply-chain teams should align with quality early, particularly under tariff pressure and broader logistics volatility. Leaders can reduce vulnerability by qualifying secondary sources where feasible, negotiating supply assurances, and building inventory policies that balance safety stock with expiry risk. Standardizing platforms across sites can simplify spare parts management, training, and service relationships, often yielding a lower operational burden even when unit prices fluctuate.
Digital readiness is now inseparable from compliance readiness. Investments in instrument connectivity, role-based access control, and audit trails can reduce manual transcription and strengthen data integrity. Equally important is governance: clear SOPs for exception handling, result review, and trending can prevent minor anomalies from becoming major deviations. Finally, capability building matters-training programs that emphasize method fundamentals, contamination control, and investigation discipline will reduce recurring errors and make automation deliver its promised gains.
A triangulated methodology combining stakeholder interviews, technical documentation review, and structured validation-focused frameworks ensures decision-relevant findings
The research methodology integrates structured primary and secondary workstreams designed to reflect real purchasing drivers, operational constraints, and compliance expectations within endotoxin testing. Secondary research establishes the baseline understanding of technologies, regulatory frameworks, compendial context, quality trends, and supplier positioning by synthesizing publicly available technical documentation, standards references, regulatory communications, product literature, and corporate disclosures.Primary research complements this foundation through interviews and consultations with stakeholders across the ecosystem, including quality control leaders, microbiology and analytical laboratory managers, validation specialists, manufacturing quality representatives, procurement professionals, and technical experts involved in endotoxin method development. These conversations focus on workflow realities such as sample matrix challenges, investigation triggers, training constraints, automation adoption barriers, and practical expectations for vendor support.
To ensure consistency, findings are organized using a defined framework that connects product categories, assay types, sample applications, end-user environments, and regional operating models. Qualitative insights are cross-checked through triangulation, comparing stakeholder feedback with documented method requirements, typical laboratory practices, and observable product capabilities. Special attention is given to factors that influence adoption, including data integrity, method transferability, supply continuity, and validation workload.
The final analysis is subjected to editorial and technical review to confirm clarity, internal consistency, and alignment with current industry direction. This approach prioritizes decision relevance by focusing on how endotoxin testing systems are selected, implemented, qualified, and sustained in real regulated environments, rather than treating technology features in isolation.
Endotoxin testing is shifting toward integrated, resilient, and digitally defensible programs as labs balance compliance rigor with faster release expectations
Medical endotoxin testing systems are evolving from standalone assays into integrated, compliance-forward capabilities that shape release speed and operational resilience. The sector’s direction is clear: laboratories want defensible results with less variability, fewer manual steps, and stronger digital traceability. This is pushing adoption of automation-ready platforms, tighter workflow standardization, and more systematic governance around data integrity and investigations.At the same time, the industry is reassessing long-standing dependence on animal-derived reagents as recombinant alternatives mature and sustainability expectations rise. The pace of change varies by product type and region, yet the underlying objectives converge around supply security, method robustness across complex matrices, and consistent performance across distributed networks.
Looking ahead, tariff-driven cost volatility and global logistics uncertainty will further elevate total cost of ownership and vendor reliability as selection criteria. Organizations that invest in harmonized platforms, validation discipline, and resilient sourcing will be better positioned to prevent release delays, reduce deviations, and maintain patient-centric quality outcomes even as external pressures intensify.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Medical Endotoxin Testing System Market
Companies Mentioned
The key companies profiled in this Medical Endotoxin Testing System market report include:- Associates of Cape Cod, Inc.
- bioMérieux
- Bio‑Rad Laboratories
- Cambrex Corporation
- Charles River Laboratories International, Inc.
- Cytiva
- Eurofins Scientific SE
- FUJIFILM Wako Pure Chemical Corporation
- GenScript Biotech Corporation
- Hyglos GmbH
- Lonza Group Ltd.
- Merck KGaA
- Microcoat Biotechnologie GmbH
- Nelson Laboratories, LLC
- Pacific BioLabs
- Pacific BioLabs
- Sartorius AG
- SGS SA
- Steris plc
- Thermo Fisher Scientific Inc.
- Toxikon Corporation
- WuXi AppTec
- Xiamen Bioendo Technology Co., Ltd.
- Zhanjiang A&C Biological Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
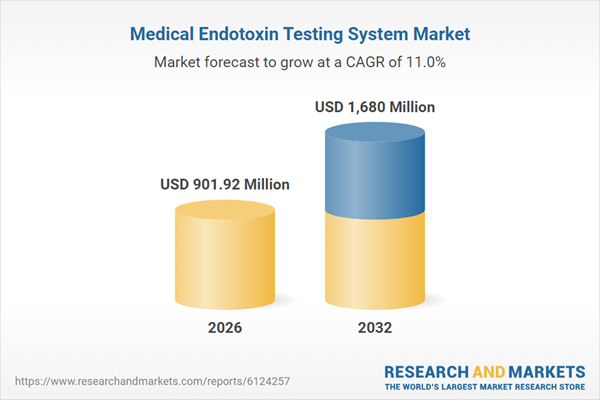
| Estimated Market Value ( USD | $ 901.92 Million |
| Forecasted Market Value ( USD | $ 1680 Million |
| Compound Annual Growth Rate | 11.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |