Speak directly to the analyst to clarify any post sales queries you may have.
Adhesives for medical wearables are now a core performance system, shaping comfort, data quality, safety, and scalable manufacturing outcomes
Medical wearables have moved from novelty to daily healthcare infrastructure, supporting continuous monitoring, therapeutic delivery, and remote patient engagement across acute and home settings. As device designs evolve toward thinner profiles, longer wear times, and more diverse user populations, the adhesive system has become a primary determinant of clinical usability and commercial success. Adhesives for medical wearable devices are no longer a “component choice” made late in development; they are a foundational design decision that influences signal quality, skin integrity, user comfort, and regulatory risk.At the same time, the adhesive interface is being asked to do more than simply “stick.” It must maintain consistent contact through sweat, motion, showers, and temperature swings while minimizing skin trauma on removal. It must also coexist with sensors, electrodes, drug reservoirs, and microfluidics without migrating, leaching, or interfering with measurements. As a result, teams increasingly evaluate adhesives as engineered systems involving chemistry, backing materials, coating uniformity, liner selection, and packaging that preserves performance over shelf life.
This executive summary frames the market landscape through the lens of what is changing fastest: patient expectations for comfort and aesthetics, the clinical push toward longer monitoring windows, and the operational pressure to scale production with high yield. It focuses on the decisions that matter most for product leaders and sourcing teams-how to align adhesive selection with wear-time targets, skin safety requirements, and supply resilience-while anticipating policy and regional shifts that can reshape cost and availability.
From “stickiness” to skin-safe engineered interfaces, wearable adhesives are shifting with longer wear, stretch electronics, and tighter quality demands
The landscape for medical wearable adhesives is undergoing a structural shift from short-duration, hospital-adjacent applications to longer-duration, consumer-like experiences that must still meet medical-grade expectations. This transition has changed the definition of “failure.” Where early wearables could tolerate occasional edge lift, newer continuous monitoring and therapy platforms often cannot; even small delamination events can introduce motion artifacts, electrode impedance variability, and user frustration that drives discontinuation.A major shift is the increasing priority placed on skin health management rather than simple adhesion strength. Manufacturers are adopting gentler formulations, redesigning tack profiles, and using breathable constructions to reduce maceration during extended wear. In parallel, the industry is becoming more sophisticated about the diversity of skin types and conditions. Pediatric, geriatric, and chronically ill populations introduce heightened sensitivity, fragile skin, and complex medication interactions that may alter skin barrier properties. This has accelerated interest in adhesives designed for atraumatic removal and consistent wear across a broad demographic spectrum.
Another transformative change is the convergence of adhesives with advanced materials engineering. The rise of stretchable electronics and conformal sensors demands adhesive layers that flex and recover without wrinkling, creeping, or creating stress concentrations at corners. Designers are also paying more attention to moisture management and vapor transmission, balancing breathability with water resistance for showering and active lifestyles. As wearables move into wellness-adjacent settings, the aesthetic expectation-low-profile, discreet, and “skin-like”-also influences backing selection and adhesive optical clarity.
Supply chain and manufacturing realities are also reshaping the landscape. Brands are seeking tighter process control over coating weight, cure profiles, and liner release consistency because small variances can cascade into assembly yield losses and field complaints. In response, converters and adhesive suppliers are investing in more application-specific product lines and validation support. At the same time, sustainability pressure is emerging in the form of reduced liner waste, solvent reduction, and packaging optimization, although these initiatives must be balanced against stringent medical quality requirements.
Finally, regulatory and clinical scrutiny is becoming more exacting as wearables become part of therapeutic decision-making. Biocompatibility expectations, chemical characterization, and change control discipline are increasingly front-and-center. This is pushing the industry toward earlier cross-functional alignment between R&D, regulatory, quality, and procurement so that adhesive choices are defensible, traceable, and resilient to inevitable supplier or geopolitical disruptions.
Tariff-driven cost, sourcing, and qualification pressures in 2025 will reshape adhesive supply chains and elevate change control as a competitive capability
United States tariff dynamics anticipated for 2025 create a layered set of impacts for medical wearable adhesive supply chains, particularly where raw materials, coated intermediates, liners, and converting services cross borders multiple times before reaching final assembly. Even when finished medical devices qualify for specific regulatory pathways and healthcare-driven exemptions, many upstream inputs remain exposed to trade policy changes, reclassification risk, and administrative complexity that can alter landed cost and lead times.One cumulative impact is the increased incentive to regionalize portions of the adhesive value chain. When tariffs affect imported films, specialty chemicals, or coated constructions, device manufacturers often reassess whether to shift converting, slitting, or die-cutting closer to final assembly to reduce cross-border movements of semi-finished goods. This can improve responsiveness but may constrain supplier options for niche chemistries and high-precision coating capabilities, especially for products requiring narrow tolerances and validated processes.
Another impact is the amplification of qualification and change-management burdens. Tariff pressure can trigger supplier substitutions-such as alternative liners, different acrylic monomer sources, or modified packaging-that appear minor but can materially affect peel, shear, breathability, and skin interaction. For regulated wearables, these substitutions can cascade into verification activities, documentation updates, and potential clinical usability risk. As a result, organizations are more likely to maintain dual-sourced bills of materials and to lock specifications earlier, even if that reduces flexibility.
Pricing volatility also becomes more difficult to manage when tariffs coincide with broader shifts in petrochemical inputs, silicone feedstocks, and logistics constraints. Adhesive programs are increasingly negotiated with a total-cost mindset that includes scrap rates, rework, packaging yields, and field performance-because small delamination-related returns can quickly overwhelm apparent per-unit savings. In this environment, procurement teams are pairing tariff scenario planning with manufacturing-focused KPIs, making supplier performance in coating consistency and lot-to-lot repeatability a strategic lever rather than a tactical detail.
Lastly, tariff-related uncertainty tends to accelerate the move toward higher transparency in origin documentation and stricter supplier auditing. Traceability expectations that were once concentrated in active device components are extending into adhesive constructions, particularly when wearables are used for clinical decisions or medication support. Companies that proactively map their adhesive supply chain, validate alternates, and design for manufacturability under multiple sourcing scenarios will be best positioned to absorb 2025 tariff shifts without compromising product performance or regulatory posture.
Segmentation insights show adhesive choices diverge by chemistry, construction, application interface, wear duration, and go-to-market validation pathways
Segmentation reveals that performance expectations diverge sharply depending on how the adhesive is used within the wearable system and what the user experiences during wear and removal. When evaluated by resin chemistry, acrylic systems remain central because they offer a versatile balance of tack, breathability potential, and formulation tunability for skin contact; however, silicone-based approaches are increasingly selected where gentle removal and high skin tolerance are paramount, especially for sensitive users and repeated application cycles. Hydrocolloid and hybrid constructions continue to play an important role in moisture management and cushioning, particularly where skin protection and comfort are prioritized, although they require careful design to avoid excessive residue or bulk. Rubber-based formulations persist in some short-wear or cost-driven contexts, yet their skin-compatibility and aging characteristics demand extra scrutiny for medical-grade use.When viewed through the lens of product type, the market separates into pressure-sensitive adhesive layers, adhesive tapes, and adhesive films that integrate bonding with functional barrier properties. Pressure-sensitive adhesive layers are increasingly engineered with defined coat weights and controlled viscoelastic behavior to reduce edge lift while preserving comfort under motion. Adhesive tapes are often chosen for their manufacturability and well-understood converting routes, enabling high-speed assembly, though they must be matched to device geometry to avoid wrinkling or stress points. Adhesive films are gaining importance as wearables become thinner and more integrated, because films can deliver consistent thickness, moisture resistance, and compatibility with automated lamination processes.
Application-based segmentation highlights that skin contact adhesives are the most scrutinized, as they sit at the intersection of biocompatibility, wear duration, and user adherence. Device-to-device bonding-such as attaching housings, batteries, or sensor modules-places greater emphasis on shear strength, temperature stability, and resistance to plasticizer migration from device polymers. Specialty uses, including electrode interfaces, drug delivery patches, and wound-adjacent wearables, introduce additional constraints around ionic conductivity, occlusivity, or interaction with active ingredients, reinforcing the need for application-specific validation.
Wear duration segmentation underscores a defining industry trend: moving from short-term to extended-wear designs without sacrificing skin integrity. Short-wear constructions can prioritize immediate tack and easy removal, while medium-wear solutions require more sophisticated moisture management to prevent maceration. Long-wear adhesives must balance higher cohesive strength with breathability and atraumatic removal, often leading teams to combine optimized adhesive chemistry with breathable backings and edge-seal strategies. End-use segmentation further clarifies decision criteria: continuous glucose monitoring and cardiac monitoring demand stability of signal and consistent contact, drug delivery wearables require predictable adhesion throughout dosing windows, and fitness or wellness wearables emphasize comfort, sweat resistance, and aesthetics-yet each still faces medical-grade expectations as devices influence health behavior.
Finally, segmentation by distribution channel and customer type illustrates how purchasing and validation pathways vary. Direct supply relationships with device OEMs tend to require deeper technical collaboration, change control commitments, and co-development support, whereas medical distributors and converters may prioritize standardization, lead-time performance, and flexibility in order quantities. Across these segments, the most successful adhesive strategies align chemistry, construction, and validation evidence with the real-world wear scenario rather than relying on generic adhesion metrics.
Regional insights across the Americas, EMEA, and Asia-Pacific reveal distinct regulatory, climate, and manufacturing drivers shaping adhesive selection
Regional dynamics in medical wearable adhesives reflect differences in regulatory expectations, healthcare delivery models, manufacturing ecosystems, and consumer adoption of wearable health technologies. In the Americas, demand is strongly influenced by large-scale remote monitoring programs, robust device innovation pipelines, and a mature ecosystem of converters and contract manufacturers. This environment favors suppliers that can support rapid design iterations, provide consistent quality documentation, and scale converting capacity for high-volume launches while maintaining tight lot traceability.Across Europe, the Middle East, and Africa, the landscape is shaped by strong emphasis on patient safety, cautious adoption cycles in certain healthcare systems, and rigorous expectations for materials transparency. Device developers often prioritize adhesives with strong biocompatibility evidence and well-controlled chemical characterization, particularly as wearables are integrated into care pathways. Additionally, multilingual labeling and diverse climatic conditions-from humid coastal regions to arid environments-drive interest in adhesives with predictable performance under varied temperature and moisture profiles.
In Asia-Pacific, rapid expansion of electronics manufacturing, growing healthcare digitization, and strong materials science capabilities are accelerating innovation in thin, conformal constructions. Many programs benefit from proximity to component supply chains for sensors, flexible circuits, and films, which can speed integration and reduce iteration time. At the same time, the region’s diversity in regulatory processes and end-user expectations means adhesive solutions often need localization, including tailoring for high-activity use cases, hot and humid climates, and differing norms around skin comfort and device discreetness.
Taken together, these regional insights point to a common strategic requirement: aligning adhesive development with regional manufacturing footprints and compliance practices. Companies that proactively design adhesive platforms adaptable to local sourcing, converting, and documentation standards can reduce launch friction and improve resilience when disruptions-whether logistical, geopolitical, or regulatory-affect cross-border material flows.
Company insights highlight differentiation through medical-grade quality systems, co-development support, and innovation in skin-safe extended-wear constructions
Competitive positioning among key companies in adhesives for medical wearable devices is increasingly defined by application specialization and the ability to support end-to-end product realization. Leaders differentiate through portfolios that span skin-contact formulations, high-cohesion bonding layers for device assembly, and specialty constructions for electrodes and drug delivery. Just as important, they provide technical services that translate lab performance into production outcomes, including guidance on surface preparation, lamination parameters, and wear testing protocols that reflect real user behavior.Another key differentiator is quality infrastructure. Companies with strong medical-grade change control, robust traceability, and disciplined management of raw material variability are better positioned to support regulated wearables that must remain consistent across long product lifecycles. This includes the capability to manage liner and backing changes, maintain coating uniformity, and document biocompatibility-relevant attributes without triggering unnecessary redesign or revalidation.
Innovation strategies also vary. Some companies prioritize chemistry innovation-such as gentler silicone systems, breathable acrylic formulations, or hybrid structures that combine skin comfort with extended wear. Others compete through converting excellence, offering precision die-cutting, complex laminations, and integrated packaging solutions that protect adhesive performance through shipping and storage. Increasingly, partnerships between formulators, film producers, and converters are used to shorten development timelines and reduce interface risk between materials.
Finally, customer collaboration has become a central competitive asset. With wearables, adhesive performance is inseparable from device geometry, mass distribution, and user interaction. Companies that co-develop with OEMs-conducting joint wear studies, iterating edge designs, and troubleshooting field feedback-tend to secure longer-term programs. As the market matures, the strongest competitors will be those that combine material science depth with manufacturing pragmatism and regulatory readiness, enabling device makers to scale confidently across regions and use cases.
Actionable recommendations focus on wear-scenario validation, manufacturability discipline, dual-sourcing readiness, and closed-loop improvement from field feedback
Industry leaders can strengthen their adhesive strategy by treating the adhesive interface as a clinical and operational risk-control point rather than a commodity input. That begins with defining wear scenarios early-activity level, showering exposure, expected reapplication behavior, and removal frequency-and translating those into measurable requirements beyond peel strength, including edge-lift resistance under flex, residue thresholds, and skin response after repeated cycles.Next, organizations should build a validation plan that mirrors real-world use and includes diverse skin profiles. Incorporating panels that reflect age, skin condition, and environmental exposure improves confidence and reduces late-stage surprises. In parallel, device teams should align adhesive choices with industrialization needs by specifying coating weight tolerances, liner release targets, and storage conditions that can be maintained across suppliers and geographies. This reduces assembly variability and helps protect sensor performance, especially for electrode-based monitoring.
Supply resilience should be addressed through structured dual-sourcing and pre-qualified alternates for high-risk inputs such as specialty liners, films, and key monomers. Rather than waiting for disruptions, leaders can use controlled design-of-experiment approaches to understand which material attributes truly drive performance, enabling smarter substitutions without full redesign. Where feasible, consolidating constructions across multiple SKUs-without compromising skin safety-can also reduce qualification burden and purchasing complexity.
Finally, leaders should invest in cross-functional governance that ties adhesive decisions to post-market feedback. Field data on edge lift, irritation reports, and user complaints should feed directly into material selection, packaging design, and instructions for use. When combined with supplier collaboration on continuous improvement, this closed-loop approach turns adhesive performance into a durable differentiator that supports retention, clinical outcomes, and brand trust.
Methodology combines value-chain interviews and technical evidence to connect adhesive material science with wearable design, quality, and supply resilience
The research methodology for this report integrates primary and secondary research to build a structured view of adhesives for medical wearable devices, emphasizing technology trends, regulatory considerations, and supply chain realities. The process begins with defining the product scope around wearable-relevant adhesive systems, including skin-contact layers, device-assembly bonding, and specialty interfaces such as electrodes and drug delivery patches. This scoping ensures that insights reflect how adhesives function within complete wearable architectures rather than as isolated materials.Primary research is conducted through interviews and structured discussions with stakeholders across the value chain, including material scientists, product engineers, quality and regulatory professionals, converters, and procurement leaders. These inputs are used to identify decision criteria, emerging pain points, and practical trade-offs observed during development and scaling. The research also captures how companies test adhesives for real-world wear conditions, manage change control, and qualify alternates under compliance constraints.
Secondary research complements these findings by reviewing publicly available technical documentation, regulatory guidance, standards frameworks relevant to skin-contact materials, patent activity signals, and corporate communications from industry participants. This evidence base supports triangulation of themes such as extended-wear performance requirements, breathable constructions, and the interplay between adhesive choice and sensor reliability.
Finally, insights are synthesized through a structured analytical framework that compares materials and strategies across applications and regions, highlighting patterns, risks, and opportunity areas. Throughout the process, quality checks are applied to ensure internal consistency, remove unsupported claims, and keep conclusions grounded in verifiable industry practices and observable market behavior.
Conclusion underscores that adhesive strategy is pivotal to wearable adoption, enabling skin safety, reliable sensing, and resilient scale-up worldwide
Adhesives for medical wearable devices sit at the critical boundary between advanced technology and the human body, making them uniquely decisive for both performance and trust. The market’s evolution toward longer wear, more diverse users, and higher clinical reliance is elevating requirements for skin safety, breathability, and consistent sensor contact. In parallel, manufacturing scale-up and policy-driven supply pressures are pushing teams to treat adhesive selection as a disciplined, cross-functional program rather than a late-stage materials decision.Segmentation and regional dynamics reinforce a central takeaway: there is no universal best adhesive, only best-fit systems aligned to wear duration, application interface, and the realities of regional manufacturing and compliance. Companies that pair material innovation with rigorous validation, strong change control, and resilient sourcing will be better positioned to reduce field issues and accelerate adoption.
As wearable healthcare continues to expand, the adhesive interface will remain a key enabler of comfort, adherence, and data reliability. Organizations that invest in adhesive strategy today-through better testing, smarter standardization, and deeper supplier collaboration-will be able to deliver devices that users keep on, clinicians trust, and manufacturers can scale with confidence.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Adhesives for Medical Wearable Device Market
Companies Mentioned
The key companies profiled in this Adhesives for Medical Wearable Device market report include:- 3M Company
- Adhesives Research, Inc.
- Avery Dennison Corporation
- Berry Global, Inc.
- Dow Inc.
- DuPont de Nemours, Inc.
- Dymax Corporation
- Elkem AS
- FLEXcon Company, Inc.
- H.B. Fuller Company
- Henkel AG & Co. KGaA
- Lohmann GmbH & Co. KG
- Nitto Denko Corporation
- Polymer Science, Inc.
- Scapa Group plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
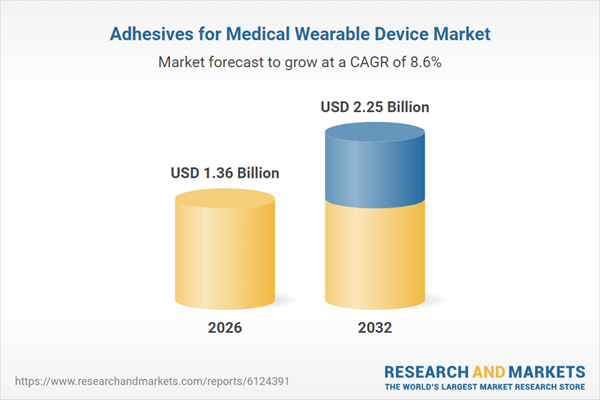
| Estimated Market Value ( USD | $ 1.36 Billion |
| Forecasted Market Value ( USD | $ 2.25 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |