Speak directly to the analyst to clarify any post sales queries you may have.
Sterile anoscopes are becoming a strategic clinical and supply choice as infection prevention, throughput demands, and outpatient migration reshape adoption
Sterile anoscopes sit at the intersection of diagnostic accuracy, patient comfort, and uncompromising infection prevention. As care teams navigate higher procedural throughput, stricter hygiene expectations, and heightened scrutiny around hospital-acquired infections, the selection of sterile anoscopes is increasingly treated as a strategic clinical and operational decision rather than a routine supply choice. In parallel, outpatient migration and the growing role of ambulatory centers are changing how these devices are evaluated, purchased, and standardized.Demand is being shaped by a practical set of pressures: clinicians want reliable visualization and ergonomic handling; facilities want consistent sterility assurance and simplified reprocessing burdens; and procurement teams want predictable availability with defensible total cost. These priorities elevate product design features such as atraumatic insertion profiles, anti-fog or clarity-supporting construction, compatibility with illumination, and packaging that supports aseptic technique.
At the same time, the sterile anoscope category is being influenced by broader shifts in medical device supply chains. The industry is balancing single-use convenience against sustainability requirements, responding to evolving regulatory expectations for labeling and quality systems, and rethinking supplier footprints to protect continuity. This executive summary frames the key forces shaping competition and adoption, the impact of trade policy headwinds, and the strategic implications for stakeholders across manufacturing, distribution, and care delivery.
From simple instrument buying to workflow optimization and resilience planning, sterile anoscope demand is being reshaped by new decision criteria
The landscape for sterile anoscopes is undergoing a pronounced transition from basic instrument procurement to outcome- and workflow-oriented purchasing. Clinicians and administrators increasingly evaluate devices through the lens of end-to-end procedure efficiency, including how quickly the instrument can be prepared, how consistently it delivers visualization, and how it supports documentation practices. As a result, product development is shifting toward usability refinements, improved material transparency, and designs that reduce glare, fogging, and handling fatigue during repetitive use.Another transformative shift is the broader normalization of single-use sterile devices in settings where reprocessing capacity is constrained or costly. Facilities are reassessing the hidden operational burdens of reusable instruments, including staff time, sterilization equipment uptime, monitoring requirements, and the risk profile associated with reprocessing variability. While reusable options remain relevant in certain environments, the preference for sterile, ready-to-use solutions is strengthening where standardized infection control and predictable turnaround time are critical.
Supply chain resilience has also become a competitive differentiator. After several years of volatility across resin availability, packaging components, and freight costs, buyers are more cautious about over-reliance on single geographies and single-source suppliers. Manufacturers are responding by adding redundant production lines, qualifying alternate materials, and strengthening quality oversight across tiers of suppliers. This shift is reinforced by provider procurement teams that increasingly request continuity plans, lead-time guarantees, and transparency about manufacturing locations.
Finally, sustainability expectations are reshaping how “sterile disposable” is justified. Stakeholders are paying closer attention to packaging reduction, recycled-content initiatives where feasible, and take-back or waste-segmentation guidance that is practical for clinical environments. The market is thus moving toward a more nuanced value proposition-one that balances sterility assurance and workflow advantages with credible environmental and compliance narratives.
United States tariff dynamics in 2025 intensify cost and sourcing pressure, pushing sterile anoscope suppliers toward redesign, diversification, and contracting shifts
United States tariffs anticipated in 2025 create a meaningful layer of uncertainty for sterile anoscopes and adjacent components, particularly where supply chains depend on imported polymers, molded subassemblies, metal parts, and sterile packaging materials. Even when finished devices are assembled domestically, tariff exposure can appear upstream through tooling, raw materials, and contract manufacturing inputs. Consequently, the tariff environment is less about a single price shock and more about compounding cost pressure that can surface across multiple points of the bill of materials.Manufacturers are likely to respond through a combination of pricing discipline and structural mitigation. In the near term, some suppliers may attempt targeted price adjustments tied to specific SKUs, packaging formats, or contract terms. However, because healthcare buyers are highly price-sensitive and increasingly sophisticated in value analysis, blanket increases can trigger competitive displacement. This dynamic favors suppliers that can document the rationale for changes, offer stable supply commitments, and preserve clinical equivalence while optimizing costs through design-to-value programs.
Over the medium term, tariff pressure accelerates supply chain reconfiguration. Dual sourcing becomes more attractive, particularly for molded components and sterile packaging that can be qualified through controlled change management. Some manufacturers will explore nearshoring or reshoring steps such as domestic molding, final packaging, or sterilization services to reduce exposure. Yet these moves require careful planning because capacity constraints, regulatory validations, and quality system harmonization can extend timelines.
For provider organizations and distributors, the cumulative impact is felt in contracting strategy and inventory posture. Buyers may seek longer-term agreements that lock in pricing and supply, while also requiring contingency commitments and visibility into country-of-origin risk. In turn, inventory buffers may increase for critical SKUs, especially in high-throughput colorectal and gastroenterology practices that cannot tolerate interruptions. Ultimately, tariffs function as a catalyst that rewards operational transparency, robust supplier qualification, and disciplined portfolio management.
Segmentation shows sterile anoscope choices hinge on design, materials, usage format, end-user workflow, and channel fit rather than price alone
Segmentation patterns highlight that product expectations differ materially depending on how sterile anoscopes are designed, packaged, and ultimately used in clinical pathways. When viewed through the lens of product type, demand often separates between devices optimized for routine anoscopy and those adapted for enhanced visualization workflows, particularly where compatibility with illumination or accessory integration is prioritized. This distinction matters because clinicians may accept different trade-offs between rigidity, field-of-view consistency, and patient comfort depending on the typical indication mix and the frequency of repeat procedures.Material-driven segmentation is equally influential because it shapes clarity, tactile feel, and perceived quality. Transparent constructions tend to be favored for straightforward visualization and teaching environments, while alternate material choices can be positioned around improved durability, reduced glare, or a more premium handling experience. In addition, the market’s increasing attention to latex-free and allergen-aware material profiles is pushing suppliers to standardize safer material declarations and improve labeling transparency.
Sterility and usage-format segmentation also creates clear decision forks. Single-use sterile configurations are frequently selected where reprocessing bottlenecks, infection-control audits, or staffing constraints make reusable inventory harder to manage. Meanwhile, facilities with mature central sterile processing may still consider reusable pathways, but they increasingly scrutinize reprocessing validation, instrument lifespan, and the operational cost of turnaround time. Across both approaches, packaging format and ease of aseptic presentation are becoming practical differentiators, especially in outpatient settings where staff multitask across rooms.
End-user segmentation underscores that the purchasing conversation changes by care setting. Hospitals tend to emphasize standardization, contract compliance, and alignment with infection-prevention committees, while ambulatory surgical centers and specialty clinics often prioritize rapid room turnover, storage efficiency, and simplified training for rotating staff. Distribution-channel segmentation further shapes adoption, as direct sales can support clinical education and trials, whereas distributor-led models compete on breadth, availability, and contract reach. Taken together, these segmentation dimensions reveal that winning strategies align product attributes with the operational realities of each use case rather than relying on one-size-fits-all positioning.
Regional adoption patterns reflect outpatient expansion, procurement centralization, regulatory stringency, and supply reliability across the Americas, EMEA, and Asia-Pacific
Regional dynamics reveal that sterile anoscope adoption is as much about care delivery models and regulatory expectations as it is about clinical need. In the Americas, purchasing decisions are heavily influenced by infection-prevention governance, group purchasing structures, and the continued shift of routine diagnostic procedures toward outpatient environments. Buyers often value suppliers that can demonstrate dependable fill rates, documentation readiness, and contract flexibility that supports multi-site standardization.Across Europe, Middle East & Africa, variation in reimbursement structures and procurement centralization shapes supplier access and product preferences. Many European markets maintain strong emphasis on regulatory compliance, traceability, and environmental considerations, which can raise expectations for packaging efficiency and lifecycle rationale. In parts of the Middle East, rapid expansion of private healthcare and new hospital builds can increase demand for standardized sterile consumables, while several African markets can face procurement constraints that prioritize durable supply arrangements and distributor reliability.
In Asia-Pacific, growth in ambulatory services and private specialty clinics often drives interest in sterile, ready-to-use devices that minimize reliance on centralized reprocessing infrastructure. At the same time, procurement can be highly heterogeneous, with some markets emphasizing premium clinical features and others focusing on consistent access and affordability within tender systems. Manufacturers that adapt to local registration requirements, language labeling, and channel structures are better positioned to scale.
Across all regions, supply chain confidence is emerging as a universal qualifier. Healthcare providers increasingly expect visibility into manufacturing footprints and contingency planning, especially where geopolitical or logistics disruptions can ripple into procedure scheduling. As regional procurement matures, suppliers that pair competitive clinical performance with operational credibility are more likely to secure long-term placement.
Company differentiation increasingly depends on sterility assurance, usability refinements, workflow support, and verifiable supply resilience under scrutiny
Competition among sterile anoscope suppliers is increasingly defined by execution across quality systems, product usability, and dependable fulfillment. Leading companies differentiate through consistent sterility assurance, validated packaging integrity, and manufacturing controls that reduce defect risk and variability across lots. Because these devices directly influence clinician confidence and patient experience, even modest improvements in optical clarity, edge finishing, and ergonomic handling can shape preference and repeat ordering.Another common differentiator is the ability to support customer workflows beyond the device itself. Companies that provide clear instructions for aseptic presentation, compatibility guidance for illumination or accessory use, and straightforward training materials reduce friction at the point of care. In environments where staff turnover is high or where procedures are performed across multiple sites, this operational support becomes a meaningful advantage.
Commercial strategy also separates competitors. Some suppliers emphasize direct engagement with colorectal and gastroenterology stakeholders to drive trials and conversions, while others leverage distributor networks to extend reach in fragmented outpatient markets. Across both models, contract readiness and responsiveness to value analysis committees matter, particularly when buyers weigh single-use sterile options against reusable alternatives and need defensible rationale.
Finally, supplier credibility is increasingly reinforced by resilience posture. Companies that can demonstrate multi-site manufacturing, alternate sourcing for key inputs, and disciplined change management are better positioned in an environment shaped by tariff uncertainty and component availability swings. As buyers ask more detailed questions about continuity, quality history, and country-of-origin exposure, the most competitive firms will be those that pair strong clinical performance with transparent, auditable operational practices.
Industry leaders should win through design-to-value, tariff-resilient sourcing, committee-ready value narratives, and pragmatic sustainability execution
Industry leaders can strengthen their position by aligning product and commercial choices with the realities of procedure rooms and procurement committees. Prioritizing design-to-value initiatives is a practical first step, focusing on features that clinicians notice immediately-smooth insertion profiles, consistent visualization, and packaging that supports clean technique-while eliminating cost drivers that do not improve outcomes or workflow. In parallel, leaders should formalize a clear rationale for single-use versus reusable portfolios, supported by operational evidence such as reprocessing burden, turnaround time, and infection-control consistency.To manage tariff-related and geopolitical risk, decision-makers should accelerate supplier diversification and qualification. This includes mapping tier-two and tier-three exposure for plastics, molded parts, and sterile packaging, then building redundancy where lead times and validation pathways allow. Establishing disciplined change control and pre-approved alternates can prevent disruption while maintaining compliance. Where nearshoring or domestic finishing is viable, leaders should evaluate phased approaches that reduce exposure without forcing abrupt transitions that could strain capacity.
Commercially, companies should tighten their value communication to match how facilities actually buy. That means preparing concise, committee-ready documentation that addresses sterility assurance, packaging integrity, ease of use, and total workflow impact. For outpatient channels, it also means ensuring distributor partners can maintain availability, provide product education, and support conversions without adding complexity to ordering. When possible, offering standardized kits or procedure-aligned configurations can simplify inventory and reduce pick errors.
Finally, leaders should proactively address sustainability expectations with credible, implementable steps. Reducing secondary packaging, optimizing carton density for shipping efficiency, and providing practical disposal guidance can help align with facility goals without compromising sterility. By integrating clinical usability, supply resilience, and responsible packaging into a coherent roadmap, organizations can compete on value rather than reacting to price pressure alone.
Methodology integrates clinician and procurement interviews with regulatory and product evidence, triangulated through segmentation frameworks for decision-ready insight
The research methodology for analyzing the sterile anoscope market blends structured primary inquiry with rigorous secondary review to ensure practical relevance and decision-grade insight. Primary research typically includes interviews with stakeholders such as clinicians involved in anoscopy procedures, infection prevention leaders, sterile processing professionals, outpatient administrators, and procurement personnel. These discussions focus on real-world selection criteria, pain points in use and handling, reprocessing considerations, and the operational drivers that influence conversion between device formats.Secondary research complements these insights through review of regulatory and standards-related materials, public company disclosures, product documentation, tender and procurement practices where available, and technical literature related to infection control and device design. Particular attention is given to how labeling, quality systems expectations, and sterilization validation intersect with adoption decisions. This step also supports a structured understanding of competitive positioning, distribution approaches, and manufacturing footprint considerations.
To translate inputs into coherent findings, the analysis applies triangulation across sources, comparing stakeholder perspectives with documented product characteristics and observable procurement patterns. Segmentation frameworks are used to organize insights by device and usage attributes, end-user settings, channels, and regional contexts. Throughout, inconsistencies are resolved through follow-up validation, and conclusions are anchored in repeatable logic rather than isolated anecdotes.
Quality assurance measures include editorial review for clarity and compliance with scope, as well as consistency checks to ensure that insights reflect current market conditions such as supply chain normalization efforts, evolving outpatient care models, and policy-driven cost pressures. This approach is designed to deliver a balanced view that supports strategic planning, product management, and commercial execution.
Sterile anoscopes are increasingly defined by infection-control priorities, outpatient workflow needs, and resilient sourcing rather than commoditized purchasing
Sterile anoscopes are gaining strategic importance as healthcare systems place greater weight on infection prevention, standardized workflow, and predictable procedure throughput. What was once treated as a relatively routine instrument category now sits within a broader discussion about operational efficiency, supply continuity, and the role of single-use sterile devices in modern care pathways.The market’s direction is being shaped by practical selection criteria that vary by setting, from hospital standardization priorities to outpatient demands for speed and simplicity. Meanwhile, trade policy uncertainty and upstream cost pressures are reinforcing the need for resilient sourcing and transparent supplier practices. These forces collectively reward manufacturers that can deliver consistent sterility assurance, usability improvements that matter at the point of care, and dependable fulfillment.
For stakeholders across the value chain, the path forward is not defined by one variable such as price or preference for disposable formats. Instead, success comes from aligning product design, packaging, and commercialization with the realities of clinical practice and procurement governance, while preparing for supply-side volatility. Organizations that integrate these priorities will be better positioned to earn clinician trust, secure contracting wins, and sustain long-term adoption.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Medical Sterile Anoscopes Market
Companies Mentioned
The key companies profiled in this Medical Sterile Anoscopes market report include:- Ambu A/S
- Boston Scientific Corporation
- Cardinal Health, Inc.
- CONMED Corporation
- FUJIFILM Holdings Corporation
- Medtronic plc
- Narang Medical
- Olympus Corporation
- Pentax Medical (HOYA Corporation)
- Smith & Nephew plc
- Teleflex Incorporated
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
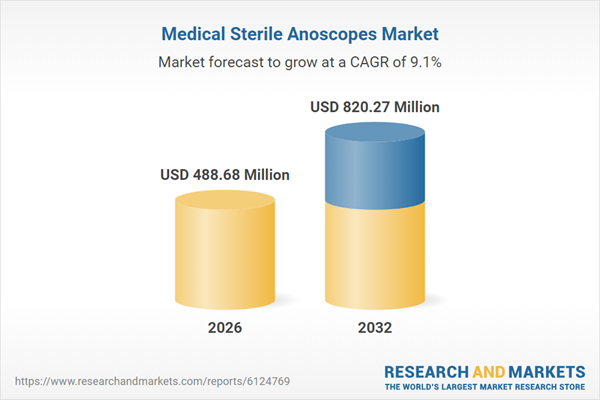
| Estimated Market Value ( USD | $ 488.68 Million |
| Forecasted Market Value ( USD | $ 820.27 Million |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |