Speak directly to the analyst to clarify any post sales queries you may have.
Precision, compliance, and scalability converge as medical stampings become a strategic enabler for modern device performance and manufacturability
Medical stampings sit at a critical intersection of precision manufacturing, regulatory discipline, and patient safety. From miniature components inside minimally invasive instruments to durable structural elements within implantable and non-implantable devices, stamped parts often determine how reliably a product performs under real clinical conditions. As OEMs continue to compress device footprints, increase functional integration, and demand higher repeatability at scale, stamping has become less of a commodity process and more of a strategic capability.What makes the category distinctive is the combination of stringent tolerances and strict material and cleanliness expectations. Manufacturers are expected to deliver consistent dimensional control, validated processes, traceable lots, and robust documentation while still meeting cost and lead-time targets. At the same time, product designers want greater freedom to iterate, pushing stampers to support early-stage prototyping, design-for-manufacturability guidance, and rapid ramp-to-volume without sacrificing quality.
Against this backdrop, the medical stampings landscape is evolving in ways that affect every stakeholder: OEMs selecting suppliers, contract manufacturers expanding vertical integration, and tooling specialists modernizing their platforms. The most competitive players are building operational resilience and technical depth simultaneously, recognizing that future advantage will come from the ability to produce complex components reliably, verify them quickly, and sustain supply under shifting global constraints.
Automation, digital quality systems, advanced tooling, and resilience planning are redefining how medical stampings are engineered, validated, and supplied
The medical stampings landscape is undergoing transformative shifts driven by device miniaturization, accelerated innovation cycles, and heightened scrutiny on quality systems. One major change is the steady move toward tighter geometric tolerances and more complex part features, including thin-gauge forms, micro-perforations, and multi-step progressive designs. As these requirements intensify, suppliers are differentiating through advanced tooling approaches, in-process measurement, and improved die protection strategies that reduce variation over long production runs.Another shift is the growing expectation for manufacturing transparency and faster validation. OEMs and critical tier suppliers increasingly require clear process capability evidence, rigorous control plans, and rapid response to deviations. This has boosted investment in digital quality management, electronic device history records, and stronger integration between inspection systems and production equipment. In parallel, automation is expanding beyond press feeding into downstream handling, packaging, and traceability marking, helping reduce human variability while improving throughput and cleanliness controls.
Materials and surface performance are also reshaping competitive dynamics. Demand continues to expand for corrosion-resistant alloys, high-strength stainless grades, and materials suited for harsh sterilization methods. As a result, the ability to manage springback, burr formation, and edge integrity has become central to meeting functional and safety expectations. Increasingly, stamping providers are pairing forming expertise with secondary operations such as deburring, electropolishing coordination, heat treatment control, or specialized cleaning validation to meet end-use constraints.
Finally, procurement strategy is changing in response to supply continuity risks. Medical stampings buyers are paying closer attention to supplier concentration, geographic exposure, and tool transfer readiness. Consequently, manufacturers that can offer dual-sourcing options, validated backup tooling plans, and disciplined capacity management are gaining trust in long-term programs. Together, these shifts are pushing the market toward higher technical intensity, stronger compliance infrastructure, and more resilient operating models.
United States tariffs in 2025 are reshaping landed-cost models, compliance rigor, and sourcing strategies, accelerating redundancy and regionalization priorities
The cumulative impact of United States tariffs in 2025 is shaping medical stampings decisions less through headline rates and more through compounding operational effects across the supply chain. When tariffs apply to metals, semi-finished inputs, tooling components, or subassemblies, the resulting cost pressure often cascades into quoting behavior, supplier selection, and inventory policy. Even when a specific finished stamped component is not directly targeted, upstream cost changes can still affect total delivered cost, especially for programs with multiple secondary processes or high material content.In response, many buyers are re-evaluating landed-cost models and tightening contract language related to price adjustments, surcharge mechanisms, and change control. Procurement teams are asking suppliers to demonstrate how they manage tariff classification, country-of-origin documentation, and compliance evidence in ways that reduce the risk of unexpected assessments. This has elevated the importance of trade compliance capabilities, including harmonized code discipline, auditable supplier declarations, and clear traceability from raw material through finished goods.
Operationally, tariffs can amplify lead-time volatility by encouraging shifts in sourcing and logistics patterns. Some manufacturers are expanding domestic processing steps or qualifying alternate material sources, which can require revalidation and additional documentation. Others are adjusting buffer stocks, which impacts working capital and warehouse controls, including lot segregation and shelf-life tracking for packaged components. These changes can be especially consequential for medical stampings, where clean handling, packaging integrity, and traceable chain-of-custody are essential.
Strategically, tariffs are accelerating a broader trend toward regionalization and redundancy. Companies are weighing nearshoring, dual sourcing, and tool localization not only to manage tariff exposure but also to improve responsiveness and reduce disruption risk. Over time, the programs most likely to remain stable are those designed with supply flexibility in mind-parts optimized for manufacturability across multiple qualified sites, materials with realistic alternates, and documentation frameworks that support controlled transitions without compromising regulatory expectations.
Segmentation insights show how materials, processes, applications, end users, and volume-precision needs shape supplier selection and capability differentiation
Segmentation reveals how medical stampings decisions are being made at the intersection of part function, manufacturing route, end-use requirements, and commercial expectations. When viewed through the lens of material type, the market’s demands emphasize predictable formability, corrosion resistance, and performance under sterilization, which places a premium on suppliers that can control springback, maintain edge integrity, and document material traceability consistently. Meanwhile, the segmentation by process type highlights that progressive die stamping remains a cornerstone for high-repeatability production, yet the complexity of geometries and tolerance stacks is increasing the value of hybrid approaches that integrate forming with secondary operations under tightly controlled work instructions.Looking at segmentation by application, the requirements diverge meaningfully based on where the stamped part ultimately performs. Components tied to surgical instruments often prioritize dimensional precision, smooth edges, and robust finishing to support repeated sterilization and safe handling, whereas device subcomponents integrated into assemblies frequently emphasize repeatable fit, controlled friction, or electrical continuity depending on the system design. This divergence is influencing how suppliers position their capabilities, with stronger emphasis on application-specific validation evidence, tailored inspection plans, and packaging configurations that protect critical-to-quality features.
Segmentation by end user further clarifies purchasing behavior and partnership models. OEMs typically seek long-term reliability, engineering support for design refinement, and disciplined change management to protect regulatory submissions. Contract manufacturers and tier suppliers may prioritize speed-to-qualification, scalability, and supplier responsiveness to engineering change orders as device platforms evolve. As a result, stampers that can flex between development support and sustained production-without weakening documentation discipline-tend to secure higher-trust relationships.
Finally, segmentation by production volume and precision requirement underscores why operational maturity matters. High-volume programs reward automation, robust preventive maintenance, and process capability discipline, while lower-volume or higher-complexity programs reward rapid tooling iteration, strong prototyping workflows, and clear feasibility communication. Across these segmentation dimensions, the common denominator is the expectation that suppliers will convert engineering intent into stable, validated manufacturing performance while protecting supply continuity and total quality cost.
Regional insights highlight how Americas, Europe, Middle East & Africa, and Asia-Pacific differ in quality expectations, scale advantages, and supply resilience needs
Regional dynamics in medical stampings reflect the balance between manufacturing heritage, regulatory expectations, and evolving supply-chain risk strategies. In the Americas, buyers often prioritize supplier responsiveness, robust quality systems, and the ability to support engineering changes quickly, particularly for programs that require rapid iteration or close collaboration between design and manufacturing teams. The region’s focus on supply continuity is also driving interest in localized capacity, validated backup plans, and transparent documentation practices that ease audits and reduce transfer friction.In Europe, the market is strongly shaped by precision engineering traditions and stringent expectations for quality management and traceability. Customers frequently emphasize process validation discipline, comprehensive technical files, and careful control of special processes tied to surface condition and cleanliness. Sustainability considerations and responsible sourcing are also increasingly present in supplier evaluations, elevating the role of material origin documentation, waste reduction practices, and efficient production methods that can be defended during stakeholder reviews.
The Middle East and Africa present a more varied landscape, where demand growth and healthcare infrastructure investment intersect with evolving local manufacturing and import dynamics. In many cases, buyers seek reliable access to validated components and may depend on established international supply chains, while simultaneously exploring regional partnerships that can shorten lead times and improve service levels. Suppliers that can support flexible logistics, strong packaging integrity, and clear compliance documentation are often better positioned to meet diverse customer expectations.
Asia-Pacific remains a critical hub for manufacturing scale and process specialization, with a strong emphasis on cost-effective production and increasingly sophisticated quality infrastructure. The region’s competitive edge often comes from high-throughput operations, deep tooling expertise, and broad supplier ecosystems for materials and secondary processes. At the same time, buyers are applying greater scrutiny to traceability, process controls, and audit readiness, which rewards suppliers that can demonstrate consistent documentation and stable process capability across multi-site networks.
Across these regions, the most durable strategies combine regional strengths with global governance: standardized validation approaches, harmonized documentation, and clear pathways for controlled changes that protect both product performance and regulatory confidence.
Company insights reveal differentiation through tooling sophistication, validated secondary operations, audit-ready quality systems, and resilient program management models
Key company strategies in medical stampings increasingly converge around technical depth, compliance maturity, and customer integration. Leading suppliers are investing in modern press platforms, progressive tooling expertise, and in-line inspection systems that enable tighter tolerances and more consistent repeatability. Just as importantly, they are strengthening upstream engineering engagement to influence part design early, reducing downstream risk tied to burrs, springback, and tolerance stack challenges.Another differentiator is the ability to manage end-to-end requirements that extend beyond the press. Many high-performing companies either provide secondary operations directly or tightly coordinate validated partners, recognizing that surface condition, edge quality, and cleanliness can be as critical as dimensional accuracy. This is reinforced by expanded clean handling practices, validated cleaning processes where applicable, and packaging controls designed to prevent damage or contamination during shipment and storage.
Quality systems are becoming a visible competitive asset rather than a background requirement. Companies that perform well in audits tend to maintain disciplined document control, strong calibration programs, robust corrective and preventive action workflows, and clear traceability that supports rapid containment if issues arise. As customer expectations rise, suppliers are also improving change management practices, ensuring tooling modifications, material substitutions, or process adjustments are evaluated, documented, and communicated in ways that protect regulatory and customer commitments.
Commercially, the strongest organizations are positioning themselves as long-term partners through program management rigor and capacity planning transparency. They are increasingly prepared with dual-sourcing narratives, tool transfer playbooks, and continuity plans that reflect the reality of geopolitical and logistics volatility. In a category where a small component can become a critical path constraint, these company-level capabilities often determine who earns preferred status in new program awards.
Actionable recommendations focus on early DFM collaboration, resilient sourcing and tooling plans, faster verification, and governance for volatility and compliance
Industry leaders can act now to reduce risk while improving performance by treating medical stampings as a design-to-supply discipline rather than a discrete fabrication step. Start by institutionalizing early manufacturability collaboration: align design, quality, and supplier engineering teams on critical-to-quality features, allowable edge conditions, and inspection strategy before the drawing is frozen. This approach helps prevent late-stage revisions that trigger revalidation, tooling churn, and avoidable delays.Next, strengthen resilience through intentional sourcing architecture. Qualify alternates where feasible, but pair that with controlled documentation that makes transitions realistic rather than theoretical. Tooling strategies should include clearly defined ownership, maintenance standards, and transfer readiness criteria, supported by documented setup parameters and inspection methods that can be replicated across sites. Where dual sourcing is not practical, negotiate transparency around capacity, preventive maintenance, and contingency plans so continuity is not left to assumptions.
Operational excellence initiatives should focus on variation reduction and verification speed. Invest in in-process controls, automated inspection where it improves repeatability, and measurement systems analysis to ensure data reliability. Additionally, treat cleanliness and packaging as engineered processes: define acceptable particulate levels where relevant, validate cleaning methods when required, and design packaging to protect edges and critical geometries throughout distribution.
Finally, align commercial models to the realities of material volatility and trade uncertainty. Use clear change control and pricing frameworks that specify how material substitutions, tariff-related cost movements, or logistics disruptions are handled. When paired with disciplined quality governance, these steps help leaders achieve a practical balance: stable supply, compliant documentation, and competitive cost structures without sacrificing speed or innovation.
Methodology integrates rigorous secondary review with stakeholder interviews and triangulation to translate technical complexity into executive-ready insights
The research methodology for this medical stampings analysis combines structured secondary research with detailed primary engagement to build a practical, decision-oriented view of the landscape. The work begins with an extensive review of public and industry-accessible information, including regulatory frameworks shaping medical manufacturing, technology developments in stamping and inspection, and documented trade and logistics considerations influencing sourcing choices.Primary research is then used to validate assumptions and capture operating realities that are not visible in public materials. This includes interviews and consultations with stakeholders such as OEM engineering and sourcing professionals, quality and regulatory leaders, tooling and operations specialists, and executives responsible for manufacturing strategy. These discussions focus on how requirements are changing, which capabilities are becoming essential, where qualification bottlenecks emerge, and how companies are responding to risk in supply continuity.
Findings are synthesized through triangulation, cross-checking inputs across multiple roles and perspectives to reduce bias and improve reliability. The analysis emphasizes consistency in definitions and terminology, particularly around process types, quality expectations, and documentation practices. Where viewpoints diverge, the methodology prioritizes explaining the conditions under which each perspective holds, helping readers understand how decisions vary by application, risk tolerance, and operating model.
Finally, the study is structured to support executive action. Insights are organized around strategic levers-technology, quality governance, sourcing resilience, and operational execution-so readers can map conclusions directly to investment decisions, supplier strategies, and program management priorities.
Conclusion underscores medical stampings as a strategic capability where precision, validation discipline, and resilient supply models determine long-term success
Medical stampings are increasingly defined by their role in enabling advanced device performance rather than simply providing cost-efficient metal components. As tolerances tighten, geometries become more intricate, and validation expectations rise, the market rewards organizations that pair tooling and process expertise with disciplined quality systems and end-to-end control of finishing, cleanliness, and packaging.At the same time, trade uncertainty and supply-chain volatility are reinforcing the need for resilience. Companies that can document traceability, manage compliance confidently, and support controlled transitions-whether through alternate sourcing, tool transfer readiness, or regional capacity options-are better positioned to protect continuity for critical programs.
Ultimately, leadership in this space comes from integrating engineering collaboration, operational rigor, and governance mechanisms that anticipate change. Stakeholders who treat stamping as a strategic capability, invest in verification speed and process control, and design supply chains for robustness will be best equipped to meet evolving customer and regulatory expectations while sustaining dependable delivery.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Medical Stampings Market
Companies Mentioned
The key companies profiled in this Medical Stampings market report include:- AIDA Engineering, Ltd.
- Bruderer AG
- China First Heavy Industries Co., Ltd.
- Hitachi, Ltd.
- JIER Machine-Tool Group Co., Ltd.
- Komatsu Ltd.
- Minster Machine Company, LLC
- Murata Machinery, Ltd.
- Schuler AG
- Sutherland Presses Ltd.
- YUTAKA GIKEN CO., LTD.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
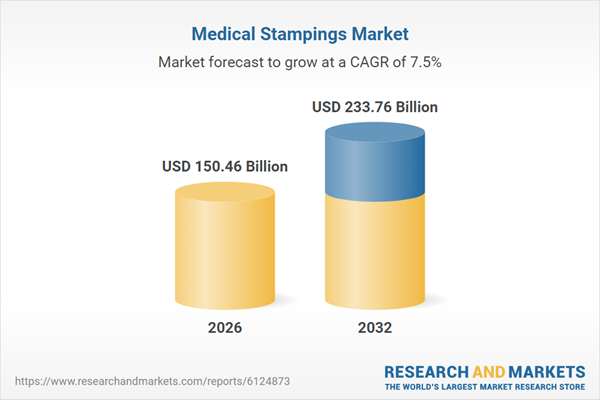
| Estimated Market Value ( USD | $ 150.46 Billion |
| Forecasted Market Value ( USD | $ 233.76 Billion |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |