Speak directly to the analyst to clarify any post sales queries you may have.
Digitizing stethoscopes are redefining frontline cardiopulmonary assessment by making auscultation shareable, analyzable, and workflow-ready
Digitizing stethoscopes is no longer a niche upgrade to a century-old instrument; it is becoming a foundational layer in modern clinical assessment. By combining acoustic capture with digital signal processing, these devices deliver cleaner sound, consistent amplification, and the ability to store and share auscultation recordings. The shift matters because auscultation often sits at the first step of cardiopulmonary evaluation, and small improvements in signal quality, reproducibility, and documentation can cascade into faster triage, more confident decision-making, and better continuity of care.What makes the current moment particularly consequential is the convergence of clinical pressure and technical maturity. Clinicians are balancing increasing patient loads, more complex comorbidities, and higher expectations for documentation, while health systems push to reduce avoidable escalation and unnecessary testing. At the same time, microphones, low-power chips, Bluetooth stacks, and on-device algorithms have advanced enough to support practical, everyday use. As a result, digitizing stethoscopes are being evaluated not simply as devices, but as endpoints in a broader ecosystem of telehealth workflows, electronic medical records, remote monitoring programs, and AI-assisted decision support.
This executive summary synthesizes how the landscape is evolving, what external forces are reshaping cost and sourcing, and where the most meaningful opportunities and constraints sit across product types, end users, and regions. It also highlights how leading companies are differentiating and where industry leaders can take immediate action to reduce deployment risk while building durable clinical and commercial advantage.
The market is moving from amplified acoustics to software-defined, interoperable, and governance-ready auscultation ecosystems
The landscape is shifting from “better sound” toward “connected clinical evidence.” Early digital models primarily competed on amplification and noise reduction, but current solutions increasingly emphasize recording, time-stamping, tagging, and secure sharing. This evolution is important because it transforms auscultation from an ephemeral bedside skill into a data asset that can be reviewed by specialists, used in training, and incorporated into longitudinal patient records.Another transformative shift is the rise of software-defined differentiation. Hardware performance is still critical, especially in challenging environments such as emergency departments or ambulances, yet competitive advantage is increasingly delivered through firmware updates, app features, algorithmic filtering tuned to lung versus heart sounds, and integrations with telehealth or documentation systems. This shift also changes buying dynamics: stakeholders now include IT, cybersecurity, and digital transformation teams alongside clinicians and procurement.
In parallel, clinical adoption is expanding beyond the stethoscope’s traditional use. Remote consultations are pushing providers to capture and transmit high-fidelity sounds from home health visits or satellite clinics. Education programs are adopting recorded libraries of murmurs and adventitious lung sounds to standardize training. Meanwhile, AI-assisted auscultation is moving from research to real-world experimentation, where algorithms support pattern recognition and prioritization rather than replace clinical judgment.
Finally, the landscape is being shaped by trust and governance. As recordings become part of patient data, questions around consent, storage, and access control become central. Health systems are more likely to scale deployments when device vendors can demonstrate secure encryption, controlled data retention policies, and auditable workflows. Consequently, companies that align product design with clinical governance and regulatory expectations are positioned to convert pilots into standardized programs.
United States tariffs in 2025 may elevate cost volatility, accelerate supplier diversification, and intensify focus on total cost of ownership
United States tariffs in 2025 are poised to influence digitizing stethoscopes through both direct and indirect pathways. Because many devices rely on globally sourced components-such as microphones, processors, batteries, and wireless modules-tariff changes can increase bill-of-material costs or introduce volatility in sourcing decisions. Even when final assembly occurs domestically or in tariff-advantaged locations, upstream exposure can still affect total landed cost and lead times.A practical impact is the renewed focus on supply chain resilience and supplier diversification. Manufacturers may respond by qualifying alternate component sources, rebalancing assembly footprints, and increasing inventory buffers for critical parts. While these steps can stabilize availability, they can also raise working capital needs and slow down product refresh cycles if engineering teams must redesign around substitute components. In turn, slower refresh cycles can delay improvements in battery life, wireless performance, or on-device processing-features that often determine clinical satisfaction.
Tariffs can also reshape contracting and pricing strategies for health systems. Procurement teams may see more frequent price revisions, shorter quote validity windows, or tiered pricing tied to volume commitments. This can make pilots more difficult to expand if budget assumptions change mid-year. As a result, vendors that can provide transparent cost drivers, lock pricing for defined periods, or offer service-based models that reduce upfront device costs may be more competitive in tariff-uncertain conditions.
Importantly, tariffs may accelerate a broader decoupling between hardware margins and software value. If hardware costs rise, vendors may emphasize subscription features such as secure cloud storage, collaboration, AI-enabled analysis, and integration services. Health systems, however, will scrutinize total cost of ownership and insist that software fees map to measurable workflow benefits. The net effect is a market that rewards disciplined packaging, clear value articulation, and operational readiness more than incremental hardware improvements alone.
Segmentation reveals adoption hinges on matching device type, connectivity, end-use workflow, and clinical application to real-world constraints
Key segmentation dynamics become clearer when the market is viewed through the lens of product type, connectivity model, end-use environment, and clinical application, as reflected in the segmentation list. Across digital and electronic stethoscopes, buyers are increasingly separating “signal quality” requirements from “data workflow” requirements. In settings where ambient noise is high and rapid assessments are constant, emphasis tends to fall on robust noise suppression and consistent performance under pressure. In contrast, consultative and longitudinal care settings often prioritize recording, annotation, and the ability to compare sounds over time.Connectivity choices also segment purchasing decisions in a meaningful way. Bluetooth-enabled models have become the practical default for pairing with smartphones and tablets, supporting mobile telehealth and flexible rounding. USB or wired transfer retains relevance in environments with strict device policies or where pairing management is burdensome, while Wi‑Fi and cloud-linked approaches are typically evaluated for enterprise deployments that need standardized governance, centralized administration, and streamlined sharing with remote specialists. As these options map differently to IT policies, the same product can be attractive to one hospital and unsuitable to another purely due to network and device-management constraints.
End-user segmentation highlights a widening decision-making circle. Hospitals often evaluate digitizing stethoscopes as part of standard-of-care consistency, training programs, and specialty workflows, while clinics and ambulatory centers may prioritize portability and ease of use. Home healthcare and telemedicine providers use digitization to bridge distance and to create a reliable handoff between visiting clinicians and supervising physicians. Emergency services and critical care environments, meanwhile, often value durability and speed, and they tend to adopt when the device improves triage confidence without complicating the encounter.
Application segmentation reinforces where near-term ROI is most defensible. Cardiology use cases benefit from clearer murmur characterization and specialist review of captured sounds, while pulmonology benefits from enhanced detection of wheezes, crackles, and other lung sound patterns across varied patient positions. General practice adoption grows when devices reduce uncertainty in routine exams and support documentation. Education and training use cases remain influential because institutions that standardize digital auscultation during training can normalize expectations among new clinicians, which in turn supports broader adoption in practice.
Across these segments, the most consistent adoption accelerator is workflow alignment: when capture, playback, sharing, and documentation feel natural within the encounter, clinicians use the device more consistently. Conversely, when segmentation needs are mismatched-such as enterprise governance demanded from a device optimized for solo practice-adoption stalls even if sound quality is excellent.
Regional adoption varies with infrastructure, governance, and care models, shaping distinct pathways across the Americas, EMEA, and Asia-Pacific
Regional dynamics differ sharply due to infrastructure readiness, reimbursement climates, procurement models, and digital health maturity, as reflected in the geography region list. In the Americas, enterprise health systems and integrated delivery networks are often positioned to scale digitizing stethoscopes through standardization programs, especially when telehealth and hospital-at-home initiatives are already operational. At the same time, procurement scrutiny is high, and vendors are expected to demonstrate cybersecurity controls and measurable workflow benefits. This combination creates strong opportunity for solutions that integrate cleanly with clinical documentation and remote consultation pathways.In Europe, the Middle East, and Africa, adoption tends to be shaped by heterogeneous health system structures and regulatory expectations that vary by country. Many markets value solutions that support cross-site collaboration and specialist access, particularly in regions where rural-to-urban referral pathways are strained. Data protection requirements and clinical governance models can be decisive, prompting preference for vendors with strong compliance posture and flexible deployment options that accommodate local storage, controlled sharing, and clear consent mechanisms.
In Asia-Pacific, momentum is often driven by the scale of care delivery and the need to extend quality assessment beyond top-tier hospitals. Large metropolitan systems can adopt enterprise approaches, while rapidly growing outpatient networks may seek mobile-first deployments. The region’s diversity means that connectivity, language localization, and device affordability materially influence adoption. In many markets, the ability to support remote consultation-whether between primary sites and tertiary centers or within expanding telemedicine models-acts as a strong catalyst for digitized auscultation.
Across regions, the common thread is that digitizing stethoscopes win when they reduce friction in clinician collaboration and elevate the reliability of frontline assessment. The differentiator is how each region operationalizes that goal: enterprise integration in some markets, flexible deployment and governance in others, and scale-efficient mobility in rapidly expanding care ecosystems.
Competition is increasingly defined by clinical-grade signal processing, interoperable software, and governance capabilities - not hardware alone
Company strategies in digitizing stethoscopes increasingly center on blending trusted clinical hardware with durable software capabilities. Established medical device brands often leverage clinician familiarity and distribution reach, positioning digital models as natural upgrades that preserve the core feel of auscultation while adding recording and noise reduction. Their advantage typically lies in reliability, support infrastructure, and the ability to navigate hospital procurement processes.At the same time, digital health-focused entrants and acoustics specialists often compete through software velocity and user experience. They tend to emphasize intuitive mobile apps, rapid sharing for remote consults, and feature iteration that responds to clinician feedback. Where they succeed, it is frequently because they treat the stethoscope as part of a workflow product-pairing, tagging, storing, and sending-rather than a standalone instrument.
Across both groups, differentiation is emerging in three areas. First is signal processing performance in real clinical environments, including the ability to reduce ambient noise without distorting diagnostically relevant frequencies. Second is interoperability, particularly how easily recordings can be attached to clinical notes or shared with specialists while maintaining auditability. Third is governance, including encryption, access control, retention settings, and administrative tooling that helps health systems standardize usage.
Partnership ecosystems are becoming more important as well. Companies are aligning with telehealth platforms, device management providers, and sometimes academic centers to validate usability and build training content. As digitized auscultation becomes more integrated, vendors that can deliver both the device and the operational playbook-training, support, and IT alignment-are more likely to progress from departmental trials to enterprise deployment.
Leaders can de-risk adoption by aligning workflows, governance, and training while packaging solutions for predictable scaling under cost pressure
Industry leaders can act now by treating digitizing stethoscopes as a program rather than a purchase. The first recommendation is to define priority workflows before selecting devices, clarifying whether the primary goal is bedside noise suppression, remote specialist collaboration, training standardization, or longitudinal documentation. When requirements are explicit, organizations can avoid overbuying features that are difficult to operationalize or underbuying governance that blocks scaling.Next, leaders should establish a joint clinical-IT evaluation path that includes cybersecurity review, mobile device management compatibility, and data handling policies. This reduces the risk of pilots failing due to late-stage objections around pairing, storage, or access controls. In parallel, it is essential to create clinician training that goes beyond “how to use the device” and instead focuses on how to capture consistent recordings, label them meaningfully, and integrate them into patient communication.
Manufacturers and solution providers should prioritize modularity and transparency in packaging. With tariff-driven cost uncertainty and rising expectations for software, clear separation of device capabilities, app features, and enterprise services can reduce procurement friction. Vendors that offer predictable lifecycle support, firmware updates, and documented integration options will be better positioned when customers expand from a small cohort to system-wide deployment.
Finally, both providers and suppliers should invest in outcomes-oriented operational metrics without overpromising automation. Tracking adoption frequency, time-to-consult for remote reviews, and documentation completeness can demonstrate value while keeping clinical accountability intact. Over time, these metrics also create the foundation for responsibly introducing AI-enabled features as decision support, ensuring the technology strengthens clinical practice rather than distracting from it.
A mixed-method approach combines stakeholder validation with structured secondary analysis to map technology, workflows, and deployment constraints
The research methodology integrates primary engagement with domain stakeholders and structured secondary analysis focused on digitizing stethoscopes as devices and as workflow enablers. Primary inputs typically include interviews and discussions with clinicians, procurement professionals, biomedical engineering teams, and digital health leaders to understand real-world pain points, adoption barriers, and feature priorities. These perspectives are used to validate how purchasing decisions are made and how requirements differ across care settings.Secondary research consolidates publicly available information such as regulatory and standards considerations, company product documentation, patent activity where relevant, clinical guideline context for auscultation practice, and broader digital health infrastructure trends. Product capabilities are assessed at the level of signal processing features, connectivity methods, software functionality, and security posture, with attention to how these elements affect usability and deployment readiness.
To build segmentation and regional insights, findings are organized across product, connectivity, end-use, and application dimensions, and then interpreted across regional healthcare delivery structures and digital infrastructure maturity. Throughout the process, consistency checks are applied to reconcile conflicting inputs and to ensure conclusions reflect practical constraints observed in clinical environments.
The resulting analysis emphasizes decision utility: it is designed to help stakeholders compare approaches, anticipate deployment challenges, and identify strategic priorities, while avoiding unsupported claims and maintaining a clear separation between observed trends and interpretive conclusions.
Digitizing stethoscopes are becoming workflow-integrated clinical endpoints, and scaling success depends on governance, usability, and resilience
Digitizing stethoscopes are evolving into connected clinical instruments that expand the value of auscultation through capture, sharing, and analysis. As the market shifts toward software-defined differentiation, success depends less on amplification alone and more on how seamlessly devices integrate into clinical workflows and governance models. This reality is pushing stakeholders to evaluate solutions through a broader lens that includes IT readiness, data stewardship, and long-term support.At the same time, external pressures such as 2025 United States tariffs can amplify cost and sourcing volatility, rewarding suppliers that build resilient supply chains and offer transparent total-cost structures. Segmentation shows that adoption varies meaningfully by device type, connectivity, end-use setting, and clinical application, while regional differences reflect infrastructure and regulatory diversity.
Organizations that treat digitizing stethoscopes as part of a care collaboration strategy-rather than a simple equipment refresh-will be best positioned to scale responsibly. The winners will be those who align device capabilities with clinician behavior, secure data practices, and operational metrics that demonstrate durable value in real care environments.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Digitizing Stethoscopes Market
Companies Mentioned
The key companies profiled in this Digitizing Stethoscopes market report include:- 3M Company
- A&D Company, Ltd.
- American Diagnostic Corporation
- AMI Corporation
- Ayu Devices Private Limited
- Cardionics, Inc.
- CliniCloud, Inc.
- Contec Medical Systems Co., Ltd.
- Edan Instruments, Inc.
- Eko Health, Inc.
- eKuore S.L.
- GF Health Products, Inc.
- HD Medical Group, LLC
- HEINE Optotechnik GmbH & Co. KG
- ICU Medical, Inc.
- Koninklijke Philips N.V.
- M3DICINE Pty Ltd
- Meditech Equipment Co., Ltd.
- Medline Industries, L.P.
- Omron Healthcare Co., Ltd.
- Rudolf Riester GmbH
- Sonavi Labs, Inc.
- StethoMe Sp. z o.o.
- Thinklabs Medical LLC
- Welch Allyn, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
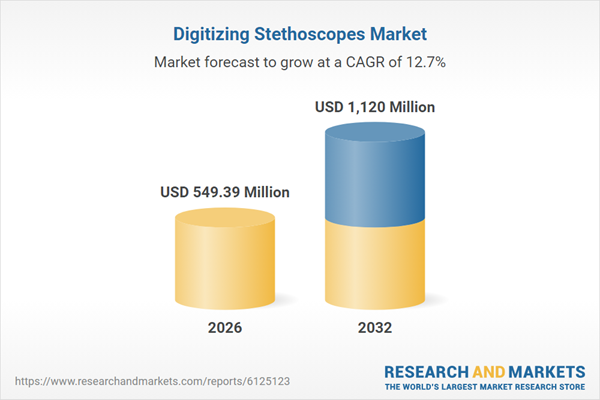
| Estimated Market Value ( USD | $ 549.39 Million |
| Forecasted Market Value ( USD | $ 1120 Million |
| Compound Annual Growth Rate | 12.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |