Speak directly to the analyst to clarify any post sales queries you may have.
Why sterile blood collection tubes are no longer mere consumables but decisive enablers of diagnostic quality and operational reliability
Sterile blood collection tubes sit at the intersection of clinical accuracy, patient safety, and laboratory efficiency. Although they appear to be standardized consumables, their performance is tightly coupled to pre-analytical integrity: additive chemistry, tube material interactions, closure integrity, draw volume accuracy, and transport stability all influence the reliability of downstream testing. As healthcare systems lean further into evidence-based medicine, high-throughput diagnostics, and tighter turnaround expectations, the tube becomes a critical control point rather than a commodity.The category is also shaped by an expanding set of use cases. Beyond routine chemistry and hematology, collection workflows increasingly support molecular diagnostics, therapeutic drug monitoring, oncology, and infectious disease panels, each placing different demands on anticoagulants, separator gels, and trace element contamination control. In parallel, decentralized testing and home-based care models continue to grow, pushing manufacturers and providers to rethink tube robustness, user instructions, and compatibility with simplified collection devices.
At the same time, procurement teams and laboratory directors are elevating expectations around quality documentation, supply continuity, and sustainability. That shift is prompting changes in how tubes are designed, validated, manufactured, and distributed. Understanding these forces is essential for stakeholders seeking to reduce redraws, prevent sample rejection, and maintain consistent performance across sites, instruments, and patient populations.
Transformative shifts redefining tube selection through automation, sustainability, clinical complexity, and a stronger resilience-first mindset
The landscape for sterile blood collection tubes is undergoing structural change driven by clinical complexity, supply-chain lessons, and rising compliance requirements. One transformative shift is the growing emphasis on pre-analytical standardization across multi-site health networks. Laboratories are harmonizing tube types, color codes, and test menus to reduce variability, but they are also demanding tighter manufacturing tolerances and stronger evidence of performance equivalency across lots. This has elevated the importance of rigorous validation data, change-control communication, and transparent quality metrics.Another shift is the acceleration of automation and workflow integration. As labs invest in automated pre-analytical lines, tubes must perform reliably under higher mechanical stress, barcode scanning, decapping/recapping cycles, and centrifugation protocols. Compatibility with analyzers, track systems, and storage solutions increasingly influences tube selection, which in turn encourages suppliers to collaborate more closely with instrument ecosystem partners. Consequently, competitive differentiation is moving toward system-level fit, not just unit cost.
Material science and sustainability pressures are also reshaping product development. Providers are scrutinizing plastics, additives, and packaging waste, while manufacturers explore resin choices, light-weighting, and recyclable secondary packaging without compromising sterility assurance or barrier properties. In addition, the need to support specialized testing is driving innovation in additive formulations, clot activators, gel separators, and tubes designed to minimize adsorption of biomarkers or drugs.
Finally, risk management has become a defining theme. After years of disruption across healthcare consumables, stakeholders are demanding dual sourcing, regional redundancy, and clearer contingency planning. This is pushing companies to reassess manufacturing footprints, supplier qualification depth, and inventory strategies. The net effect is a market that rewards operational resilience and regulatory discipline as much as product performance.
How United States tariffs in 2025 compound through inputs, documentation, and redesign efforts to reshape costs and sourcing priorities
The cumulative impact of United States tariffs in 2025 is best understood through how costs, sourcing decisions, and compliance burdens cascade across the sterile blood collection tube value chain. Tariffs affecting plastics, rubber components, medical-grade packaging, machinery, or upstream chemical inputs can translate into higher landed costs even when final assembly occurs domestically. For many suppliers, the most immediate effect is margin compression followed by selective price adjustments, particularly on high-volume tube formats where competitive pricing is tight and contracts are renewed on fixed schedules.Over time, procurement strategies are likely to shift from a narrow focus on unit price toward a broader total-cost and continuity framework. Healthcare providers and distributors may prioritize suppliers with diversified sourcing, domestic or nearshore assembly options, and proven inventory buffers. In parallel, manufacturers may increase efforts to qualify alternate raw-material sources, renegotiate long-term supply agreements, and redesign components to reduce exposure to tariffed categories while preserving performance specifications and regulatory filings.
Operationally, tariffs can increase administrative friction. Classification, country-of-origin documentation, and audit readiness take on greater importance, especially for organizations with complex multi-tier supplier networks. Any change in material or supplier can trigger additional verification and validation activities under quality system requirements, which extends timelines and elevates internal testing workloads.
Strategically, tariffs can accelerate investment in regional manufacturing redundancy and automation to offset higher labor and input costs. They can also intensify collaboration between suppliers and large laboratory networks to stabilize specifications and reduce unexpected change events. The cumulative effect in 2025 is not merely higher costs; it is a rebalancing of how stakeholders value resilience, transparency, and controllable compliance risk in a category where failure has immediate clinical consequences.
Key segmentation insights revealing why tube performance, additives, materials, and end-user workflows create a portfolio-driven market reality
Segmentation illuminates why sterile blood collection tubes behave like a portfolio market rather than a single product category, because clinical intent and workflow constraints vary across settings and test types. By product type, tubes with serum separator formats, plasma tubes with anticoagulants, EDTA tubes for hematology, citrate tubes for coagulation, heparin tubes for chemistry applications, and specialized trace element or glucose-stabilizing designs each compete on different performance attributes such as clotting time, gel stability, platelet activation control, or analyte preservation. This differentiation matters because a tube that performs well in one assay pathway can introduce subtle bias in another, making cross-validation and standardization central to customer decision-making.By material, the dynamics between plastic and glass are shaped by safety, logistics, and analytical requirements. Plastic continues to align with breakage reduction and lighter shipping, while glass may persist in niche workflows where specific chemical inertness or vacuum stability is preferred. Yet material selection is no longer purely technical; it is also influenced by transport stress, automation compatibility, and sustainability narratives that push suppliers to justify resin choices and packaging design.
Additive segmentation is increasingly influential because laboratories are expanding menus that are sensitive to pre-analytical variation. Clot activators, separator gels, anticoagulants, and preservative chemistries affect yield and stability over time and temperature excursions. As more samples are transported across hub-and-spoke networks or processed in centralized facilities, stability claims and real-world robustness can outweigh historical preferences.
From an end-user perspective, hospitals, diagnostic laboratories, blood banks, and ambulatory or outpatient centers bring distinct procurement and usage patterns. Large hospitals and integrated delivery networks often prioritize standardization, multi-site harmonization, and service responsiveness, while independent labs may emphasize compatibility with existing instrumentation and reliable distributor fulfillment. Blood banks place heightened focus on sterility assurance, regulatory compliance, and traceability, whereas ambulatory sites value ease of use and reduced error risk during phlebotomy.
Finally, distribution channel segmentation highlights how purchasing behavior evolves under disruption. Direct sales can strengthen technical support and change-control communication, while distributor and group purchasing arrangements can drive standardization and price discipline. As a result, suppliers that align technical, regulatory, and service capabilities with the expectations embedded in each segment are better positioned to sustain adoption even when contracts cycle or workflows change.
Key regional insights explaining how infrastructure, regulation, and supply continuity priorities shape adoption across global healthcare ecosystems
Regional dynamics for sterile blood collection tubes reflect differences in healthcare infrastructure, regulatory expectations, and supply-chain resilience priorities. In the Americas, demand is strongly tied to high testing volumes, extensive laboratory automation, and an emphasis on standardization across large health systems. This environment tends to reward suppliers that demonstrate consistent lot-to-lot performance, robust technical documentation, and dependable fulfillment, especially when providers are managing consolidated laboratory networks and centralized testing strategies.Across Europe, the Middle East, and Africa, purchasing decisions often balance cost controls with stringent quality and compliance requirements, alongside varied maturity in laboratory infrastructure. Western European markets typically emphasize harmonized standards, sustainable procurement practices, and strong post-market surveillance responsiveness, while parts of the Middle East prioritize capacity expansion and reliable access to premium consumables. In many African markets, supply continuity, training support, and fit-for-purpose designs that tolerate transport and storage constraints can be decisive factors.
In Asia-Pacific, the landscape is shaped by expanding diagnostic access, rapid growth in private laboratory chains, and significant investment in healthcare modernization. Large urban centers often demand automation-ready tubes and broad product ranges, while emerging areas may prioritize affordability and reliable distribution coverage. The region also includes manufacturing powerhouses, which can influence competitive intensity and shorten supply chains for certain components, although regulatory diversity requires careful localization of labeling, claims, and quality documentation.
Taken together, regional insight underscores that go-to-market strategy cannot be uniform. Suppliers that tailor portfolio emphasis, service models, and compliance readiness to regional operating realities are more likely to secure long-term adoption and withstand short-term disruptions in trade, logistics, or policy.
Key company insights on competing through quality systems, resilience, automation compatibility, and differentiated additive-driven performance
Competitive positioning in sterile blood collection tubes increasingly hinges on the ability to deliver consistent quality at scale while supporting evolving laboratory workflows. Leading companies differentiate through broad portfolios that cover routine and specialized applications, paired with quality systems capable of tight process control, sterility assurance, and disciplined change management. As laboratories become more sensitive to pre-analytical variability, suppliers that can provide clear validation support, technical education, and reliable documentation gain influence in procurement decisions.Another defining capability is operational resilience. Companies with diversified manufacturing footprints, qualified secondary sources for critical components, and mature supplier management programs are better prepared to navigate fluctuations in raw material availability and shifting trade conditions. In addition, those that invest in packaging engineering, barcode and labeling accuracy, and automation compatibility can integrate more smoothly into high-throughput environments.
Innovation is also moving beyond incremental tube variations. Firms that develop additive chemistries designed for analyte stability, reduce interference risks, and support newer diagnostic modalities can capture demand in advanced testing pathways. At the same time, sustainability programs are becoming more visible, with suppliers exploring material reductions, optimized logistics, and responsible waste considerations while maintaining strict performance requirements.
Overall, company insight points to a competitive arena where procurement teams increasingly select partners based on total value: technical performance, compliance confidence, service responsiveness, and the ability to support uninterrupted clinical operations.
Actionable recommendations to reduce pre-analytical risk, harden supply resilience, and align tube choices with automation and governance goals
Industry leaders can strengthen their position by treating sterile blood collection tubes as a strategic element of diagnostic quality management rather than a line-item expense. Prioritizing structured change control is essential: establish clear protocols for evaluating supplier notifications, validating equivalency, and communicating updates to phlebotomy and lab staff. This reduces the risk of unexpected sample integrity issues that cascade into redraws, delayed results, or clinician distrust.Next, leaders should operationalize resilience through supplier and footprint strategies. Qualifying secondary sources for high-risk components, stress-testing inventory policies against transport disruption scenarios, and negotiating supply agreements that include transparency on upstream sourcing can improve continuity. Where feasible, aligning tube selections across sites can simplify training and reduce variability, but harmonization should be paired with periodic performance audits to ensure that standardization does not mask emerging issues.
To keep pace with automation, organizations should assess tube compatibility with pre-analytical systems, centrifugation protocols, and barcode requirements as part of procurement, not after deployment. This includes verifying mechanical robustness, cap performance, and label readability under real workflow conditions. In parallel, sustainability goals should be translated into measurable specifications-such as packaging reductions or recyclable materials-without compromising sterility and barrier properties.
Finally, leaders should invest in cross-functional governance that connects procurement, quality, laboratory operations, and clinical stakeholders. When decisions reflect both cost discipline and clinical risk management, organizations are better positioned to sustain performance through regulatory shifts, trade changes, and evolving diagnostic demand.
Research methodology built on value-chain mapping, stakeholder validation, and rigorous triangulation to produce decision-ready tube insights
The research methodology is designed to translate complex clinical, operational, and regulatory factors into practical insights for decision-makers. The process begins with a structured framing of the sterile blood collection tube ecosystem, mapping how raw materials, additive chemistries, manufacturing controls, sterilization approaches, packaging, distribution, and end-user workflows interact to influence performance and risk.Primary research emphasizes stakeholder perspectives across the value chain, including laboratory operations, procurement, quality and regulatory professionals, distribution participants, and product specialists. These engagements focus on real-world decision criteria such as change-control expectations, automation compatibility, common failure modes, and supplier performance under disruption. Insights from these discussions are used to test assumptions and refine the interpretation of observed market behavior.
Secondary research consolidates publicly available technical and regulatory information, including standards, guidance documents, recall and safety communications where applicable, corporate disclosures, and product documentation. This step supports triangulation and helps ensure that conclusions align with current compliance expectations and technology directions.
Finally, the analysis applies an integrated synthesis approach. Information is cross-validated across sources and evaluated for consistency, timeliness, and relevance to sterile collection workflows. The goal is to produce decision-ready narratives and frameworks that support strategy, sourcing, and product planning without relying on speculative sizing claims.
Conclusion highlighting why pre-analytical integrity, resilience, and policy-driven sourcing shifts are redefining sterile tube strategy
Sterile blood collection tubes are becoming more strategically important as diagnostics expand, automation increases, and tolerance for pre-analytical variability declines. The category’s evolution is shaped by technical requirements-additive performance, material interactions, and sterility assurance-alongside operational imperatives such as continuity of supply, disciplined change control, and compatibility with automated laboratory environments.Transformative shifts are pushing stakeholders to reassess what “quality” means in practice. It now includes transparency, documentation readiness, and resilience under disruption, not just basic conformance. Meanwhile, trade policy dynamics in 2025 add momentum to supplier diversification and design choices that reduce exposure to upstream cost and compliance volatility.
Organizations that respond with stronger governance, deeper validation discipline, and more resilient sourcing strategies will be better positioned to protect clinical outcomes and operational performance. In this environment, informed tube selection and supplier partnership become key levers for reducing risk and sustaining trust in diagnostic results.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Sterile Blood Collection Tube Market
Companies Mentioned
The key companies profiled in this Sterile Blood Collection Tube market report include:- AdvaCare Pharma, Inc.
- Becton, Dickinson and Company
- Biosigma S.p.A.
- Cardinal Health, Inc.
- CML Biotech (P) Ltd.
- DWK Life Sciences GmbH
- F.L. Medical Srl
- Greiner Bio-One International GmbH
- Improve Medical Technology Co., Ltd.
- Narang Medical Limited
- Nipro Medical Corporation
- QIAGEN N.V.
- Sarstedt AG & Co. KG
- Sekisui Medical Co., Ltd.
- Terumo Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
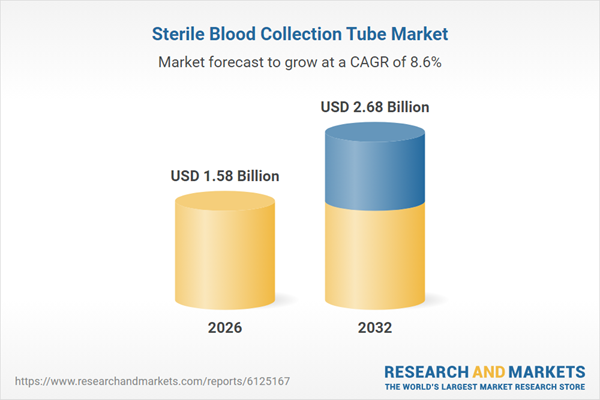
| Estimated Market Value ( USD | $ 1.58 Billion |
| Forecasted Market Value ( USD | $ 2.68 Billion |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |