Speak directly to the analyst to clarify any post sales queries you may have.
Why tucatinib tablets sit at the center of HER2-positive treatment evolution, access scrutiny, and supply-chain resilience planning
Tucatinib tablets have become a reference point in the ongoing evolution of HER2-directed oncology care, particularly where clinical decision-making must balance durable systemic control with meaningful intracranial activity. As treatment pathways for HER2-positive malignancies expand and sequencing becomes more nuanced, stakeholders across the value chain are reassessing how targeted therapies fit into increasingly personalized regimens. This has elevated the importance of consistent product quality, dependable supply, and evidence generation that reflects real-world patient complexity.At the same time, the market environment surrounding tucatinib tablets is shaped by a convergence of forces: accelerating biomarker-driven diagnosis, broader adoption of next-generation sequencing, and heightened expectations from clinicians and patients for therapies that preserve quality of life while addressing metastatic burden. These drivers are interacting with payer scrutiny, evolving guidelines, and practical constraints in oncology clinics, ultimately influencing prescribing patterns and demand variability.
Against this backdrop, decision-makers must understand not only clinical differentiation, but also operational feasibility-how manufacturing footprints, trade policy, and distribution models affect continuity of care. This executive summary frames the most consequential shifts, the emerging implications of United States tariffs in 2025, and the segmentation and regional dynamics that will shape commercial and access outcomes for tucatinib tablets.
Transformative shifts redefining tucatinib tablets through CNS priorities, combination sequencing, and supply resilience as a competitive advantage
The tucatinib tablets landscape is undergoing transformative shifts driven by scientific progress and system-level pressures that are changing what “competitive advantage” means in oncology. A key inflection is the growing clinical and operational emphasis on central nervous system involvement, where treatment selection is increasingly judged by intracranial effectiveness and the practicality of keeping patients on therapy. As clinicians prioritize regimens that can manage brain metastases while maintaining tolerability, differentiation is moving beyond response rates to include treatment continuity, adverse-event management, and the real-world feasibility of multi-agent protocols.In parallel, the expansion of HER2 testing and more precise patient stratification are reshaping diagnosis-to-treatment timelines. Earlier detection of HER2-driven disease and clearer identification of HER2 status in metastatic settings are accelerating therapy initiation and intensifying expectations for rapid payer authorization. This is prompting manufacturers and channel partners to invest more in patient support infrastructure, reimbursement navigation, and data packages that speak to outcomes in heterogeneous patient populations, including those historically underrepresented in trials.
Another notable shift is the growing role of combination strategies and sequencing logic. Tucatinib’s place in therapy is increasingly evaluated relative to antibody-drug conjugates, other tyrosine kinase inhibitors, and novel biologics that are competing for adjacent lines of care. This competition is not purely clinical; it is also defined by regimen complexity, required monitoring, drug-drug interaction considerations, and the burden placed on oncology practices. Consequently, commercial strategy is being reoriented toward simplifying adoption-through education, pathway integration, and evidence that helps clinicians choose confidently within crowded algorithms.
Finally, supply chain resilience has moved from a back-office topic to a strategic differentiator. The industry’s post-pandemic recalibration has heightened sensitivity to single-source dependencies for active pharmaceutical ingredients, excipients, and packaging components. For tucatinib tablets, where continuity matters in chronic administration, stakeholders are placing greater value on redundancy, geographic diversification, and proactive risk management. These structural changes set the stage for how tariffs and trade policy developments may amplify operational challenges and influence pricing and availability decisions.
How United States tariffs in 2025 compound cost, sourcing complexity, and access decisions for tucatinib tablets across the value chain
The cumulative impact of United States tariffs in 2025 is best understood as a chain reaction that extends beyond headline import costs, touching procurement strategy, supplier qualification timelines, and the total cost of ensuring uninterrupted therapy. For tucatinib tablets, tariffs affecting pharmaceutical inputs, key chemical intermediates, or packaging materials can translate into higher landed costs and more complex sourcing decisions. Even when finished-dose products are not directly targeted, upstream exposure can still influence manufacturing economics and create ripple effects across contract manufacturing and component procurement.One immediate implication is intensified pressure to map and document supply chains at a deeper tier than many organizations historically maintained. Manufacturers and their partners are increasingly expected to identify country-of-origin exposure for active ingredients, critical reagents, and specialized packaging, then build mitigation plans that satisfy both internal governance and external stakeholder expectations. This may accelerate dual sourcing, nearshoring initiatives, or strategic inventory buffers, but each option carries trade-offs in working capital, quality assurance capacity, and time-to-implement.
Tariffs also interact with payer and provider dynamics in ways that can subtly shape access outcomes. When costs rise, organizations may attempt to offset pressure through contracting strategies, channel optimization, and operational efficiencies. However, oncology payers and pharmacy benefit stakeholders are simultaneously emphasizing affordability and utilization management. This creates a narrow corridor where supply and pricing decisions must protect patient access while preserving sustainable margins, especially when therapy continuity is essential and switching costs-clinical and administrative-are high.
Over time, the 2025 tariff environment may further encourage localization of certain manufacturing steps, including secondary packaging or final release activities, to reduce exposure and shorten lead times. Yet localization is not a simple fix; it requires technology transfer planning, validation batches, regulatory alignment, and quality system integration. For tucatinib tablets, the strategic response is therefore likely to be portfolio-specific, blending targeted diversification with scenario-based planning that anticipates policy volatility and prioritizes uninterrupted availability for patients.
Segmentation insights that clarify where tucatinib tablets win or stall across dosage expectations, indications, channels, and end-user realities
Segmentation insights for tucatinib tablets reveal that competitive pressure and operational priorities vary markedly depending on how the market is viewed through product, application, distribution, and end-user lenses. By dosage strength, decision-making often centers on aligning prescribing flexibility with inventory efficiency, particularly as clinicians adjust dosing in response to tolerability considerations and concomitant therapies. Strength availability and presentation can influence formulary confidence, pharmacy stocking behavior, and the ease of maintaining patients on therapy without interruption.By indication, demand characteristics are shaped by line of therapy, prevalence of CNS involvement, and evolving guideline placement within HER2-positive treatment algorithms. In HER2-positive breast cancer, the therapy conversation increasingly incorporates brain metastases management and sequencing against antibody-drug conjugates and other targeted agents. In other HER2-expressing tumors where HER2 biology and testing patterns differ, adoption hinges on the consistency of diagnostic confirmation, the availability of supporting clinical evidence, and the degree of specialist familiarity with HER2-directed regimens.
By distribution channel, differences in patient touchpoints drive practical requirements for reimbursement support and adherence management. Hospital pharmacies and specialty pharmacies often play distinct roles in initiation and continuity, with specialty channels typically emphasizing prior authorization navigation, patient outreach, and shipment coordination that reduces missed doses. Retail and other channels, where present, can be constrained by handling requirements, payer routing, and the need for specialized counseling, making channel strategy an important lever for reducing abandonment and improving persistence.
By end user, the operational realities of oncology care settings become decisive. Large hospital systems and academic centers may adopt therapies rapidly when pathway committees validate the evidence and operational workflows are defined, while community oncology practices may place greater emphasis on regimen simplicity, manageable toxicity profiles, and predictable reimbursement. Consequently, manufacturers that tailor education, service models, and evidence communication to each end-user environment are better positioned to support consistent utilization without creating undue administrative burden.
Regional insights showing how reimbursement design, diagnostic capacity, and distribution readiness shape tucatinib tablets adoption worldwide
Regional dynamics for tucatinib tablets reflect how clinical capacity, reimbursement design, and supply-chain infrastructure collectively shape access and utilization. In the Americas, adoption is strongly influenced by guideline alignment, payer utilization controls, and the operational sophistication of specialty pharmacy networks. The United States adds another layer of complexity through shifting policy signals and tariff-related sourcing considerations, while Canada and Latin American markets may face distinct constraints related to centralized procurement, coverage breadth, and diagnostic access that can delay therapy initiation.Across Europe, the Middle East, and Africa, heterogeneity is the defining theme. Western European markets often combine robust diagnostic infrastructure with stringent health technology assessments and negotiated access pathways, making evidence packages and real-world outcomes especially influential. In parts of the Middle East, expansion of oncology centers and investment in advanced care can support adoption, but access may still hinge on tender frameworks and the availability of specialized distribution. In several African markets, constrained diagnostic capacity and fragmented supply logistics can limit consistent availability, elevating the importance of partnerships that support testing, education, and reliable distribution.
In Asia-Pacific, growth in precision oncology capability is accelerating, yet the region remains highly varied in reimbursement maturity and the pace of guideline incorporation. High-income markets may emphasize clinical differentiation and real-world performance, while emerging markets often balance oncology innovation with affordability constraints and tiered access. Across the region, local manufacturing policies, import procedures, and cold-chain-adjacent handling expectations-even for oral solids-can influence lead times and continuity, making operational planning as critical as clinical messaging.
Taken together, these regional patterns underscore that successful strategy cannot be exported unchanged. Stakeholders must adapt to how each region organizes oncology care, funds specialty medicines, and manages distribution, while also anticipating policy-driven disruptions that can affect sourcing and supply reliability.
Key company insights highlighting differentiation through evidence strategy, supply reliability, and pathway integration in a crowded HER2 ecosystem
Company dynamics in the tucatinib tablets space are characterized by a blend of originator-led lifecycle management and a broader ecosystem of partners that influence access, distribution, and evidence generation. The most influential players distinguish themselves through the strength of their clinical narrative in HER2-positive disease, their ability to support complex combination regimens, and their investment in services that reduce friction for prescribers and patients. This includes robust medical education, toxicity management resources, and reimbursement support that helps practices navigate prior authorization and appeals.Another differentiator lies in manufacturing and quality governance. Companies that can demonstrate consistent supply, proactive risk controls, and transparent quality systems are better positioned to maintain formulary confidence-particularly when external shocks such as tariffs or logistics disruptions raise stakeholder concerns. Contract manufacturing relationships, backup suppliers for key inputs, and disciplined change-control practices become strategic assets in a category where interruptions can undermine adherence and outcomes.
Data strategy is also separating leaders from laggards. As payers and providers request evidence that reflects real-world populations, companies are prioritizing observational studies, pragmatic data collaborations, and outcomes communication that addresses CNS disease, treatment persistence, and patient-reported experience. In parallel, partnerships with diagnostics stakeholders and oncology networks help expand appropriate testing and ensure eligible patients are identified early enough to benefit.
Finally, competitive positioning is increasingly shaped by how effectively companies navigate a crowded HER2 therapeutic ecosystem. Success depends on clearly articulating where tucatinib tablets fit relative to other modalities, enabling confidence in sequencing decisions, and supporting pathway integration without overburdening clinical workflows. Organizations that align medical, access, and operations around this unified value proposition are more likely to sustain durable adoption.
Actionable recommendations for leaders to protect access, reduce operational friction, and future-proof tucatinib tablets strategy amid volatility
Industry leaders should prioritize supply-chain risk management as a near-term strategic imperative rather than a compliance exercise. This means conducting tier-two and tier-three supplier mapping for active ingredients, excipients, and packaging, then building mitigation pathways that include qualified alternates and clear triggers for inventory buffering. Where tariff exposure is plausible, scenario planning should connect trade policy changes to procurement actions, quality timelines, and communication plans for customers and internal stakeholders.In tandem, leaders should strengthen the access and services layer that surrounds tucatinib tablets, recognizing that operational simplicity often determines whether a therapy is used consistently in real practice. Investing in prior authorization support, benefits verification tools, and patient adherence programs can reduce delays and abandonment. Just as importantly, education must be tailored to the realities of different care sites, with practical guidance on managing adverse events and coordinating combination therapy monitoring.
Evidence generation should focus on the questions decision-makers are asking now, especially around intracranial outcomes, durability in heterogeneous populations, and real-world treatment persistence. Leaders can accelerate credibility by partnering with oncology networks, registries, and academic collaborators to produce transparent analyses that align with clinical endpoints valued by guideline committees and payer reviewers. As competition intensifies, the ability to translate evidence into clear sequencing guidance-without overclaiming-will be essential.
Finally, commercial and medical teams should align around pathway integration as a core growth lever. Rather than relying solely on awareness-building, leaders should support tumor boards, pathway committees, and digital clinical decision support tools with concise, practice-ready materials. By reducing cognitive and administrative load for clinicians, companies can improve appropriate utilization while reinforcing trust in both the therapy and the organization behind it.
Research methodology built to connect clinical evidence, policy signals, and real-world access mechanics into decision-ready tucatinib tablets insights
The research methodology underpinning this executive summary is designed to translate complex clinical, policy, and operational signals into decision-ready insights for stakeholders in the tucatinib tablets ecosystem. The approach begins with structured secondary research, including review of regulatory communications, policy developments relevant to trade and tariffs, clinical guideline updates, and peer-reviewed literature focused on HER2-positive malignancies and CNS disease. This foundation supports a coherent view of how standards of care and access expectations are evolving.Primary research is then used to validate assumptions and capture practical realities that are not fully visible in published sources. Interviews and structured discussions with stakeholders such as oncologists, hospital pharmacists, specialty pharmacy leaders, payer and reimbursement experts, and supply-chain professionals help clarify how decisions are made at the point of care and within procurement organizations. These perspectives are synthesized to identify where friction occurs, what evidence is most persuasive, and how operational constraints influence prescribing and dispensing.
Analytical triangulation is applied to reconcile differences across sources and to ensure conclusions are consistent with observed market behavior. This includes cross-checking policy interpretations with supply-chain feasibility, aligning clinical adoption narratives with access mechanics, and assessing how distribution models influence adherence and persistence. Throughout, emphasis is placed on traceable logic, neutrality, and relevance to strategic decisions rather than headline metrics.
Finally, quality control steps-including consistency checks, terminology normalization, and internal peer review-are used to ensure clarity and usability. The result is a methodology that connects scientific context with commercial and operational implications, enabling stakeholders to act with confidence in a rapidly shifting environment.
Conclusion tying together therapy evolution, tariff-driven operational risk, and context-specific adoption factors for tucatinib tablets
Tucatinib tablets occupy an important position in HER2-positive oncology care at a time when therapeutic choice is increasingly shaped by CNS considerations, sequencing complexity, and the practical realities of delivering multi-agent regimens. As stakeholders raise expectations for real-world performance and continuity, the factors that determine success extend well beyond clinical differentiation to include services, channel execution, and supply reliability.The landscape is also being reshaped by macro forces that can quickly change operating assumptions. United States tariffs in 2025 exemplify how policy can compound costs and sourcing complexity, prompting deeper supply-chain visibility and more robust mitigation planning. For companies and healthcare stakeholders alike, resilience planning is becoming inseparable from patient access and brand trust.
Segmentation and regional insights reinforce a central theme: adoption is context-dependent. Dosage and presentation influence pharmacy operations, indications vary in evidence and testing readiness, channels determine adherence support capacity, and end-user environments shape workflow fit. Regionally, differences in reimbursement, diagnostics, and distribution require strategies calibrated to local realities.
Ultimately, organizations that integrate evidence, access enablement, and operational readiness into a single, coherent strategy will be best positioned to support patients and sustain performance in the evolving tucatinib tablets market.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Tucatinib Tablets Market
Companies Mentioned
The key companies profiled in this Tucatinib Tablets market report include:- Alembic Pharmaceuticals Limited
- Amneal Pharmaceuticals, Inc.
- Aurobindo Pharma Limited
- Cadila Healthcare Limited
- Cipla Limited
- Dr. Reddy's Laboratories Ltd.
- Glenmark Pharmaceuticals Ltd.
- Hetero Labs Limited
- Jubilant Generics Limited
- Lupin Limited
- Macleods Pharmaceuticals Ltd.
- Mylan N.V.
- Natco Pharma Limited
- Pfizer Inc.
- Seagen Inc.
- Strides Pharma Science Limited
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Torrent Pharmaceuticals Ltd.
- Zydus Lifesciences Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
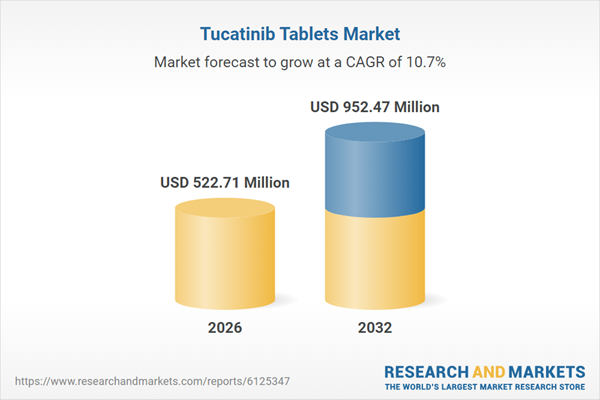
| Estimated Market Value ( USD | $ 522.71 Million |
| Forecasted Market Value ( USD | $ 952.47 Million |
| Compound Annual Growth Rate | 10.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |