Speak directly to the analyst to clarify any post sales queries you may have.
Intensive care monitoring is evolving into connected clinical infrastructure, redefining how multi-parameter systems are chosen and deployed
Multi-parameter intensive care monitors sit at the operational core of modern critical care, translating high-frequency physiologic signals into actionable clinical context. As ICU acuity rises and staffing models evolve, these monitors are increasingly expected to do more than display numbers; they must support rapid recognition of deterioration, enable consistent documentation, and integrate cleanly into the broader digital ecosystem of the hospital. In parallel, clinicians and biomedical engineering teams are demanding reliability under continuous use, intuitive workflows that reduce cognitive load, and alarms that are clinically meaningful rather than disruptive.This market is being shaped by a convergence of clinical and technical priorities. Hospitals are standardizing monitoring fleets to simplify training and maintenance, while also expanding coverage into step-down units and specialized environments that require adaptable configurations. At the same time, the procurement conversation has shifted from “device features” to “system performance,” with emphasis on interoperability, cybersecurity, service continuity, and lifecycle cost. These expectations are amplifying competition among established manufacturers and newer entrants that emphasize software, connectivity, and analytics.
Against this backdrop, multi-parameter ICU monitoring is no longer a standalone equipment purchase; it is a critical infrastructure decision that touches patient safety, clinician efficiency, and enterprise IT governance. Understanding the forces reshaping demand and the practical tradeoffs across segments and regions is essential for suppliers, providers, and investors seeking resilient strategies in a rapidly modernizing care environment.
From bedside devices to software-defined, cyber-secure, interoperable platforms, ICU monitoring is being reshaped by workflow and IT realities
The landscape is undergoing a shift from hardware-centric differentiation to software- and workflow-led value. Vendors are investing heavily in user interfaces, alarm logic, and clinical decision support features that help clinicians prioritize attention amid increasing data volume. As a result, competitive advantage is often determined by how well a monitoring platform reduces nuisance alarms, supports rapid parameter customization, and integrates seamlessly with bedside workflows rather than by raw sensing capability alone.Interoperability has become a decisive battleground. Hospitals expect multi-parameter monitors to communicate smoothly with electronic health records, central stations, anesthesia information systems, and telemetry networks. In practice, this means stronger emphasis on standards-based data exchange, vendor-neutral connectivity, and integration toolkits that reduce the burden on hospital IT teams. As health systems expand and consolidate, enterprise-wide standardization favors solutions that can scale across campuses and support consistent policy enforcement.
Cybersecurity and device governance are reshaping purchasing criteria in parallel. With connected bedside devices now treated as endpoints within hospital networks, buyers are scrutinizing patch management, authentication, encryption, and auditability. Vendors that can demonstrate disciplined secure-by-design practices and predictable update pathways are better positioned in environments where risk committees increasingly influence clinical technology procurement.
Another transformative shift is the rising role of remote and centralized monitoring models. Whether driven by staffing pressures, specialty coverage gaps, or the pursuit of more consistent surveillance, hospitals are adopting centralized command centers that rely on dependable data streams and robust alert routing. This has expanded the importance of networking performance, uptime guarantees, and support services that keep systems stable under continuous load.
Finally, supply chain resilience and service continuity have moved into the foreground. Buyers are evaluating not only device availability but also parts continuity, field service depth, and training capacity. In this environment, vendors are differentiating through service-level commitments, modular upgrade paths, and flexible financing structures that reduce disruption over the monitor lifecycle.
United States tariff dynamics in 2025 amplify supply chain and contracting pressures, reshaping pricing, sourcing resilience, and upgrade strategies
United States tariff dynamics in 2025 create cumulative effects that extend beyond headline duties, influencing pricing structures, sourcing decisions, and contract negotiations across the ICU monitoring value chain. Multi-parameter monitors and their ecosystems depend on a broad bill of materials-displays, semiconductors, sensors, cables, batteries, and network components-many of which are globally sourced. When tariffs touch upstream components or finished assemblies, suppliers face cascading cost pressure that may not be fully absorbed without impacting margins, service pricing, or bundling strategies.Over time, tariffs can intensify the push toward dual sourcing and regionalization of manufacturing. Vendors may respond by qualifying alternative suppliers, shifting final assembly locations, or increasing local content where feasible. While these actions reduce exposure, they often introduce short-term complexity, including revalidation requirements, supplier audits, and documentation updates to satisfy quality management systems. For ICU-grade equipment, where safety and reliability standards are stringent, any change in component sourcing can trigger extensive verification work.
Procurement behavior also changes under sustained tariff uncertainty. Health systems may seek longer price locks, negotiate more aggressive service inclusions, or pursue multi-year framework agreements to stabilize total cost. This can reward vendors with strong contracting discipline and transparent price justification. At the same time, smaller providers may delay refresh cycles or pursue refurbished fleets when capital budgets tighten, increasing competitive pressure in mid-tier segments.
Tariffs can further influence innovation roadmaps by shifting investment priorities. If hardware costs rise or remain volatile, manufacturers may emphasize software-enabled upgrades, subscription-based analytics, and service differentiation to preserve value without frequent hardware redesign. Conversely, tariff-driven supply volatility can encourage platform modularity so components can be swapped with minimal system disruption.
The cumulative impact is a market that becomes more sensitive to total delivered cost, lead times, and service guarantees. Companies that can demonstrate resilient sourcing, clear compliance documentation, and dependable delivery performance are likely to earn greater trust from risk-aware buyers navigating complex fiscal and operational constraints.
Segmentation highlights diverging needs across device types, parameter tiers, care settings, end users, and buying channels shaping monitor selection
Segmentation reveals that the market is best understood through how clinical use cases and purchasing models intersect. By product type, bedside monitors are increasingly evaluated as modular hubs that can expand parameter sets as acuity changes, while portable and transport monitors are prioritized for battery performance, rapid attachment, and durable form factors that tolerate frequent movement between departments. Central monitoring stations, meanwhile, are being assessed as enterprise visibility tools, with expectations for scalable licensing, reliable networking, and alarm management that aligns with hospital governance.When viewed by parameter capability, basic monitoring configurations tend to remain relevant in cost-sensitive environments or lower-acuity settings, yet even these buyers now expect upgrade paths that avoid full replacement. Mid-range systems differentiate through flexibility-adding invasive blood pressure, capnography, or advanced hemodynamic modules as needed-while high-end configurations are often selected where complex cases demand continuous, high-fidelity surveillance. Across these tiers, the purchasing decision is increasingly shaped by how well the platform supports standardized protocols and reduces training variability.
Acuity and application segmentation also clarifies buyer priorities. Adult ICUs commonly emphasize broad interoperability and alarm governance across larger fleets, whereas neonatal and pediatric environments prioritize measurement accuracy at small physiological ranges, gentle sensor design, and workflow features tailored to weight-based dosing and specialized alarms. Operating rooms and post-anesthesia care units place additional focus on rapid transitions, integration with anesthesia systems, and efficient documentation workflows that do not interrupt perioperative cadence.
End-user dynamics sharpen these differences. Hospitals often pursue enterprise standardization and centralized surveillance, while ambulatory surgical centers evaluate monitoring based on throughput, compact footprints, and simplified maintenance. Specialty clinics and long-term care or step-down environments may prioritize ease of use and reliability over maximal parameter breadth, yet still require dependable connectivity for documentation and escalation pathways.
Finally, distribution and purchasing channels shape adoption velocity. Direct sales models are common where complex integration and service commitments are essential, while distributor-led approaches can accelerate penetration in fragmented provider markets when local support and logistics matter most. Across segments, the strongest value propositions combine clinical relevance with lifecycle simplicity: scalable software, predictable service, and a clear path from initial deployment to future capability expansion.
Regional adoption patterns reflect disparities in infrastructure, regulation, service capacity, and digital maturity across global critical care ecosystems
Regional dynamics show how ICU monitoring adoption is shaped by infrastructure maturity, reimbursement environments, regulatory expectations, and workforce realities. In the Americas, replacement cycles and standardization across large health systems drive demand for platforms that integrate tightly with enterprise IT and support centralized monitoring models. Buyers often scrutinize cybersecurity posture and service responsiveness, and they increasingly expect vendors to provide strong implementation support that reduces downtime during fleet transitions.Across Europe, the Middle East, and Africa, purchasing patterns reflect a mix of highly digitized hospital networks and capacity-building markets. Western Europe typically emphasizes compliance, interoperability, and evidence-based alarm governance, while parts of the Middle East prioritize rapid hospital expansion and premium ICU build-outs that favor comprehensive monitoring solutions with strong service coverage. In many African markets, cost constraints and maintenance capacity influence decisions toward robust systems with accessible consumables and straightforward servicing, often paired with training programs to build biomedical engineering capability.
In Asia-Pacific, growth is closely tied to expanding critical care capacity, urban hospital modernization, and the scaling of digital health infrastructure. Advanced markets within the region often demand sophisticated integration, telemetry coordination, and analytics-driven workflows, while emerging markets may prioritize dependable baseline monitoring with upgrade paths that match staged capital investment. Across the region, the availability of local technical support and dependable supply of accessories can be as decisive as device specifications.
These regional contrasts reinforce a central theme: success depends on aligning product configuration, service design, and partnership models to local operating constraints. Vendors that tailor implementation, training, and lifecycle support to regional realities are better positioned to win long-term relationships, especially as health systems pursue standardized care pathways and consistent monitoring quality across diverse facilities.
Competition centers on platform breadth, interoperability, cybersecurity discipline, and service reliability as vendors defend and expand ICU footprints
Key companies in this market compete on a combination of clinical credibility, platform breadth, integration capability, and service depth. Large diversified medical technology manufacturers leverage extensive installed bases, long-standing relationships with hospital leadership, and broad portfolios that span bedside monitoring, central stations, connectivity software, and complementary ICU equipment. This breadth supports bundled contracting and standardized deployments, particularly in multi-hospital systems seeking procurement simplicity and consistent training.At the same time, specialist monitoring firms and technology-forward challengers are carving out opportunities by focusing on usability, flexible modularity, and differentiated connectivity. Their strategies often emphasize rapid deployment, cloud-enabled data services, and integration toolkits that address the practical realities of mixed-vendor hospital environments. In competitive tenders, these players may position themselves as faster-moving partners that can tailor workflows and reporting to local clinical preferences.
Service and lifecycle support increasingly separate leaders from followers. Companies that deliver predictable field service coverage, strong biomedical training, and resilient parts availability gain credibility in environments where uptime is non-negotiable. Additionally, vendors that offer structured cybersecurity maintenance-clear patch timelines, documentation support, and coordinated risk management-are strengthening their standing with procurement committees that include IT and security stakeholders.
Across the competitive field, product roadmaps are converging around interoperability, alarm intelligence, remote surveillance readiness, and modular upgrades. The companies best positioned for sustained relevance will be those that can translate these themes into measurable operational value for hospitals while maintaining reliability and compliance across diverse care settings.
Industry leaders can win by optimizing interoperability, lifecycle resilience, contracting flexibility, and clinically meaningful alarm and workflow improvements
Industry leaders should prioritize platform strategies that reduce friction for both clinicians and IT teams. Strengthening interoperability through standards-based connectivity, well-documented APIs, and proven integrations with leading clinical systems can shorten sales cycles and reduce implementation risk. Equally important is designing configuration governance so hospitals can standardize alarm policies, parameter sets, and user permissions across units without excessive customization overhead.Operational resilience should be treated as a product feature, not merely an operations function. Leaders can mitigate tariff and supply volatility by expanding dual sourcing, qualifying alternates for high-risk components, and adopting modular designs that allow substitutions without broad redesign. In parallel, transparent lifecycle planning-roadmap visibility, upgrade options, and service continuity commitments-helps buyers justify enterprise standardization decisions.
Commercial strategies should align with how providers budget and measure value. Flexible contracting that blends capital purchase, service bundles, and software subscriptions can meet varied procurement constraints while preserving long-term relationships. However, pricing architecture must remain clear and defensible, with explicit delineation of what is included in cybersecurity maintenance, integration support, and training.
Finally, leaders should invest in evidence-based workflow improvements that address alarm fatigue and clinician burden. Enhancing signal quality, refining alarm algorithms, and enabling role-based escalation pathways can deliver tangible clinical and operational benefits. When these capabilities are paired with strong implementation programs and ongoing education, vendors can shift from being device suppliers to being long-term partners in critical care transformation.
A structured methodology blends primary stakeholder validation with rigorous secondary review to capture real ICU monitoring procurement and usage realities
This research methodology combines structured secondary analysis with primary validation to ensure findings reflect real-world purchasing and deployment behavior in ICU monitoring. The process begins with systematic review of publicly available regulatory information, product documentation, company disclosures, standards guidance, and procurement-related materials to establish a grounded view of technology capabilities and compliance expectations.Primary inputs are gathered through interviews and consultations with stakeholders across the value chain, including clinical users, biomedical engineering, hospital IT, procurement leaders, and industry participants involved in manufacturing, distribution, and service. These discussions are used to validate adoption drivers, pain points, and evaluation criteria, with particular attention to interoperability, cybersecurity, service models, and upgrade pathways.
Segmentation and regional analyses are developed by mapping how needs differ by care setting, parameter requirements, purchasing channels, and local operating constraints. Insights are cross-checked through triangulation, comparing perspectives across stakeholder groups to reduce bias and to identify areas of consensus versus divergence.
Quality control is maintained through iterative review, consistency checks, and careful separation of verified observations from interpretive assessments. The result is a decision-oriented narrative that emphasizes practical implications for product strategy, go-to-market execution, and procurement planning in multi-parameter intensive care monitoring.
The market’s direction favors secure, interoperable, serviceable monitoring ecosystems that improve workflows while managing cost and operational risk
Multi-parameter intensive care monitoring is transitioning into a connected, governed, and security-sensitive layer of hospital infrastructure. The most important changes are not confined to new sensors or higher screen resolution; they are rooted in how monitoring systems integrate with clinical workflows, reduce alarm burden, support centralized surveillance, and comply with modern cybersecurity expectations.As tariffs and supply uncertainties influence sourcing and contracting, providers are placing greater emphasis on reliability, lead times, and lifecycle transparency. This environment rewards vendors that can demonstrate resilient supply chains, disciplined quality processes, and strong service continuity.
Segmentation and regional differences remain central to strategy. What succeeds in an enterprise hospital network prioritizing interoperability may not translate directly to a cost-constrained setting that values robustness and maintainability. Companies and providers that align configurations, service models, and implementation support to the realities of each segment and region will be better equipped to improve care delivery while managing operational risk.
Ultimately, the path forward favors solutions that deliver measurable workflow improvements, dependable integration, and long-term upgrade flexibility. Stakeholders that act on these priorities can build monitoring ecosystems that support safer, more efficient critical care across diverse clinical environments.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Multi-parameter Intensive Care Monitor Market
Companies Mentioned
The key companies profiled in this Multi-parameter Intensive Care Monitor market report include:- Bionet America, Inc.
- BrainScope Company, Inc.
- Cairn Research Ltd.
- Cardinal Health, Inc.
- CONTEC Medical Systems Co., Ltd.
- Drägerwerk AG & Co. KGaA
- Edan Instruments, Inc.
- Fukuda Denshi Co., Ltd.
- GE Healthcare
- Hill-Rom Holdings, Inc.
- Masimo Corporation
- Mindray Medical International Limited
- Natus Medical Incorporated
- Nihon Kohden Corporation
- Nonin Medical, Inc.
- Philips Healthcare
- Schiller AG
- Siemens Healthineers AG
- Spacelabs Healthcare
- Stryker Corporation
- ZOLL Medical Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
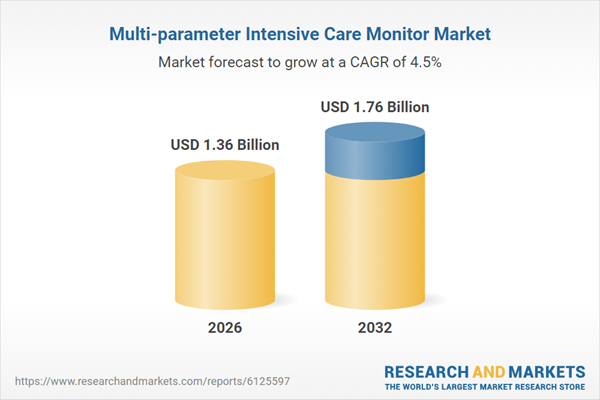
| Estimated Market Value ( USD | $ 1.36 Billion |
| Forecasted Market Value ( USD | $ 1.76 Billion |
| Compound Annual Growth Rate | 4.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |