Speak directly to the analyst to clarify any post sales queries you may have.
A high-stakes, highly regulated blood bank ecosystem is evolving quickly as safety expectations, demand volatility, and technology modernization converge
Blood banking sits at the intersection of clinical urgency, highly regulated manufacturing-like discipline, and community-based donor engagement. It is also an industry where quality failures carry immediate patient safety implications and where small inefficiencies can cascade into shortages, expiries, or delayed care. As health systems pursue tighter utilization management and as clinical pathways become more data-driven, the blood bank function is increasingly expected to deliver reliability, transparency, and responsiveness across the entire vein-to-vein chain.At the same time, the environment is becoming more complex. Patient demographics are shifting demand across components, elective care rebounds can collide with seasonal donation volatility, and pathogen and emerging-infection preparedness continues to reshape testing expectations. Against this backdrop, leaders are looking for clearer visibility into how technology, regulation, supply chain constraints, and competition are changing the operating model of blood banks.
This executive summary frames the blood bank landscape through the lens of strategic change: what is transforming, where pressure points are emerging, and how stakeholders can align investments in collection, testing, and inventory practices with near-term resilience and long-term modernization. The goal is to support decisions that improve availability and safety while strengthening cost discipline and operational agility.
Digital traceability, automation, utilization stewardship, and resilience planning are redefining how blood banks operate and compete under pressure
The landscape is being reshaped by a decisive shift toward end-to-end digital traceability. Blood banks and transfusion services are moving beyond basic inventory counts toward interoperable systems that can reconcile donor data, collection events, testing results, component processing, and transfusion outcomes. This shift is driven by the need to reduce manual reconciliation errors, improve recall readiness, and support quality audits with defensible, time-stamped records. As adoption accelerates, organizations are prioritizing integration with hospital information systems and laboratory information systems to minimize workflow friction.In parallel, automation is moving from “nice to have” to operational necessity. Automated component processing, barcoding and scanning at every handoff, and more standardized testing workflows are being adopted to counter staffing constraints and variability in technician experience. This is not merely a labor substitution trend; it is also a quality strategy. When throughput must rise quickly-during trauma surges, seasonal demand peaks, or disaster response-automation helps maintain consistency in testing and labeling while reducing rework.
Another transformative shift is the growing emphasis on patient blood management and utilization stewardship. Hospitals are refining transfusion thresholds and optimizing surgical blood ordering practices, which changes the demand pattern blood banks must serve. Instead of uniform, predictable drawdowns, many facilities see sharper peaks tied to specific service lines and more emphasis on targeted component use. Consequently, blood banks are strengthening demand planning with clinical partners and redesigning distribution strategies to reduce wastage and ensure the right component mix.
Finally, the sector is seeing heightened attention to resilience against biological and logistical disruptions. The continued focus on pathogen reduction, rapid response to emerging infectious risks, and contingency planning for transportation interruptions is pushing organizations to diversify sourcing, formalize mutual aid agreements, and invest in flexible cold-chain capabilities. As these shifts compound, competitive differentiation increasingly comes from reliability, data transparency, and the ability to adapt operations without compromising safety.
Tariffs anticipated in 2025 could reshape blood bank procurement, capital refresh decisions, and supply chain resilience through higher costs and longer lead times
United States tariff actions planned for 2025 are expected to exert uneven pressure across the blood bank value chain, particularly where specialized consumables and capital equipment rely on global manufacturing networks. While blood itself is locally collected and not “imported” in the conventional sense, the tools that enable collection, processing, testing, and storage often cross borders multiple times before reaching the point of use. As a result, even modest tariff changes can translate into higher landed costs for critical disposables, replacement parts, and select analyzers.The near-term operational impact is likely to appear first in procurement cycles and service contracts. Blood centers and hospital-based blood banks may encounter higher pricing or longer lead times for items such as blood bags, leukoreduction filters, reagents, plastics used in apheresis kits, and components of cold-chain systems. In response, many organizations will revisit supplier qualification strategies and increase dual-sourcing where feasible. However, qualifying alternative suppliers in a regulated environment is not instantaneous, particularly for products tied to validated processes.
Over the medium term, tariffs can influence technology adoption decisions. When capital equipment becomes more expensive or unpredictable in availability, organizations often extend asset life, prioritize refurbishment, or delay upgrades, which can slow modernization. Conversely, some stakeholders may accelerate investments in automation and digital systems that reduce consumable intensity, limit waste, and improve labor efficiency, effectively offsetting higher input costs. This creates a bifurcated market environment where the most strategically aligned operators use cost shocks as a catalyst for redesign.
Tariff dynamics also create indirect pressures on collaboration across the ecosystem. Contract testing arrangements, shared services, and regional distribution partnerships become more attractive when individual organizations face constrained budgets. Ultimately, the cumulative impact of tariffs in 2025 is likely to amplify the importance of supply chain governance, scenario-based procurement planning, and total-cost-of-ownership analysis rather than price-per-unit purchasing.
Segmentation insights show divergent priorities across products, applications, end users, and technologies as blood banks balance safety, speed, and cost
Segmentation reveals a blood bank ecosystem where priorities and constraints differ sharply depending on product focus, operational setting, and workflow intensity. In the product dimension, the balance between whole blood and blood components continues to shape how organizations allocate resources across processing, storage, and distribution. Component-centric strategies demand tighter inventory orchestration and more refined matching of demand to platelet, plasma, and red cell availability, while whole blood programs require clear clinical protocols and reliable cold-chain discipline to preserve usability.From an application standpoint, transfusion medicine remains the operational center of gravity, yet the surrounding applications increasingly influence investment. Therapeutic apheresis, immunohematology support, and specialized testing needs can elevate the importance of high-throughput analyzers and rapid turnaround workflows. As clinical pathways evolve, facilities that treat complex oncology, transplant, or trauma cases often emphasize readiness and redundancy, whereas smaller institutions prioritize standardized processes and outsourced support where appropriate.
End-user segmentation highlights the practical differences between hospital blood banks, standalone blood centers, and diagnostic laboratories. Hospital blood banks tend to optimize for immediate availability, tight integration with clinical systems, and bedside safety checks, while blood centers prioritize donor recruitment, collection efficiency, and regional distribution performance. Diagnostic laboratories, including reference labs, emphasize analytical throughput, quality management, and specialized assay capability, sometimes serving as central hubs that reduce duplication across multiple hospitals.
Technology segmentation underscores the divergent modernization pathways. Organizations investing in automated immunohematology platforms and advanced screening tools are typically targeting error reduction, faster crossmatching, and better surge capacity. Meanwhile, facilities upgrading cold storage, transport monitoring, and traceability systems often pursue waste reduction and compliance resilience. Across these segments, purchasing decisions increasingly hinge on interoperability, validated performance, service support reliability, and the ability to sustain operations amid staffing constraints.
Regional insights reveal distinct maturity levels and operational priorities across the Americas, Europe Middle East & Africa, and Asia-Pacific blood ecosystems
Regional dynamics in the Americas reflect a mix of advanced infrastructure and persistent variability in donor participation and rural access. The United States and Canada continue to emphasize compliance rigor, system interoperability, and patient blood management programs, while parts of Latin America focus on expanding collection capacity, improving testing standardization, and strengthening cold-chain reliability. These differences influence which technologies gain traction and how quickly centralized distribution models mature.In Europe, Middle East & Africa, regulatory alignment and public health systems shape procurement and operational models, but the region is far from uniform. Western Europe tends to prioritize harmonized quality frameworks, advanced testing, and digital traceability, while some markets in Eastern Europe, the Middle East, and Africa concentrate on scaling basic safety standards, improving donor screening, and addressing infrastructure gaps in storage and transport. As cross-border collaboration expands in select areas, resilience planning and standardized data practices become more important.
Asia-Pacific presents a wide spectrum of maturity and demand drivers, ranging from highly advanced urban hospital networks to emerging systems focused on expanding access. Large population centers with growing surgical volumes and chronic disease burdens place sustained pressure on collection and inventory management. In parallel, modernization efforts increasingly center on automation, improved donor management, and the adoption of more consistent testing protocols. Across the region, public-private collaboration and investments in logistics often determine how quickly capabilities scale.
Across all regions, leaders are converging on a shared operational truth: availability depends on coordinated networks, not isolated facilities. Regional differences therefore matter most in how they shape partnerships, technology priorities, and the pace of standardization.
Company strategies increasingly center on integrated diagnostics, consumables, software, and service models that deliver interoperability, uptime, and compliance trust
The competitive environment in blood banking spans equipment manufacturers, diagnostics and reagents providers, software and traceability vendors, and service partners that support collection and distribution. Company strategies increasingly converge around integrated offerings that reduce handoffs, simplify validation, and improve end-to-end visibility. Vendors that can demonstrate interoperability across analyzers, middleware, inventory systems, and hospital platforms are positioned to become strategic partners rather than transactional suppliers.Diagnostics and screening providers continue to focus on higher throughput, improved sensitivity, and operational simplification, reflecting the need to maintain safety while handling variable volumes. At the same time, consumables and collection-focused companies are emphasizing kit ergonomics, donor experience, and reliability of supply-attributes that become decisive when staffing constraints and donor retention challenges intensify. Service and maintenance capabilities are also gaining prominence, as downtime risk in high-utilization environments can quickly translate into delayed transfusions or increased wastage.
Software and data-centric firms are shaping differentiation through traceability, compliance reporting, and analytics that support utilization management. Systems that can surface near-expiry risk, guide redistribution, and support electronic crossmatching workflows are increasingly valued. Across categories, companies that invest in cybersecurity, validated cloud deployment options, and clear change-management support are better aligned with hospital IT governance and quality expectations.
Overall, competitive advantage is increasingly tied to trust: proven performance, regulatory readiness, dependable supply, and the ability to support customers through workflow redesign rather than isolated product upgrades.
Actionable recommendations focus on supply resilience, workflow automation, interoperability-first technology roadmaps, and data-driven donor and inventory strategies
Industry leaders can strengthen resilience by treating supply chain governance as a clinical risk domain rather than a back-office function. This means expanding dual-sourcing where validation allows, mapping tier-two and tier-three supplier dependencies, and establishing predefined substitution pathways for critical consumables and reagents. In parallel, contracting should emphasize service-level transparency, parts availability commitments, and clear escalation processes to reduce operational surprises.Operationally, organizations should prioritize workflow designs that reduce manual steps and make quality easier to sustain. Investments in automation, barcode scanning, and closed-loop traceability are most effective when paired with standardized operating procedures and role-based training that supports rapid onboarding. Additionally, leaders should align inventory policies with utilization management programs, using data to reduce expiries and create trigger-based redistribution between sites.
Technology roadmaps should be built around interoperability and validated change management. Rather than deploying isolated systems, leaders should require integration with laboratory and hospital platforms, audit-ready reporting, and configurable rules that reflect local protocols. Where cloud adoption is feasible, governance should address cybersecurity, access control, and business continuity so that digital gains do not introduce new risk.
Finally, donor and community strategies deserve the same analytical discipline as clinical operations. Strengthening donor retention through tailored communications, improving appointment management, and enhancing donation experience can stabilize supply. When paired with regional partnerships and mutual-aid arrangements, these actions help ensure that availability is maintained even when demand spikes or collection falters.
A rigorous methodology combining stakeholder interviews, regulatory and technical documentation review, and iterative triangulation supports dependable conclusions
The research methodology integrates structured primary engagement with rigorous secondary review to build a cohesive view of the blood bank ecosystem. Primary inputs typically include discussions with stakeholders across donor centers, hospital transfusion services, laboratory leadership, procurement teams, and industry participants spanning diagnostics, consumables, and software. These conversations are used to validate operational realities, identify emerging pain points, and test assumptions about adoption barriers and decision criteria.Secondary research consolidates publicly available regulatory guidance, standards, product documentation, clinical and operational publications, and corporate disclosures to contextualize trends and verify technology capabilities. This stage focuses on mapping workflow patterns across collection, testing, processing, storage, and distribution, while also capturing how compliance requirements and quality practices influence procurement and operations.
Triangulation is applied by cross-checking insights from different stakeholder groups and reconciling discrepancies through iterative validation. The analysis emphasizes consistency, reproducibility, and practical relevance, ensuring that strategic conclusions are grounded in how blood banks actually operate. Throughout, the approach prioritizes clarity on definitions and scope so readers can translate findings into actionable decisions aligned with their specific operating environment.
The blood bank sector’s next phase will reward integrated modernization that improves traceability, resilience, and clinical alignment without compromising safety
Blood banking is undergoing a period of operational redesign driven by digital traceability, automation, utilization stewardship, and heightened resilience requirements. These forces are not independent; they reinforce one another by shifting expectations toward measurable quality, faster turnaround, and supply reliability despite staffing and logistics constraints. Organizations that treat these changes as an integrated transformation-rather than a series of incremental upgrades-are better positioned to sustain patient care continuity.Tariff-related pressures expected in 2025 add urgency to modernization choices, pushing leaders to evaluate total cost of ownership, supplier risk, and the value of interoperability. In this environment, the most durable strategies are those that reduce waste, standardize workflows, and strengthen collaboration across regional networks.
Ultimately, success in blood banking will be defined by trust and performance: the ability to deliver safe products, document every step, respond quickly to disruptions, and align supply with clinical need. Stakeholders that invest in resilient processes and integrated technology will be best equipped to meet rising expectations without compromising safety or efficiency.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Blood Bank Market
Companies Mentioned
The key companies profiled in this Blood Bank market report include:- Abbott Laboratories
- Asahi Kasei Corporation
- B. Braun Melsungen AG
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- Blood Bank Computer Systems
- Cordlife Group Limited
- Cryo-Cell International, Inc.
- Danaher Corporation
- FamiCord Group
- Fresenius SE & Co. KGaA
- GE Healthcare
- Grifols, S.A.
- Haemonetics Corporation
- Hoffmann-La Roche AG
- J Mitra & Company Pvt. Ltd.
- Johnson & Johnson
- Macopharma SAS
- MAK-SYSTEM
- MedTech Solutions
- Medtronic plc
- Nipro Corporation
- Oracle Corporation
- Terumo Corporation
- WellSky Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
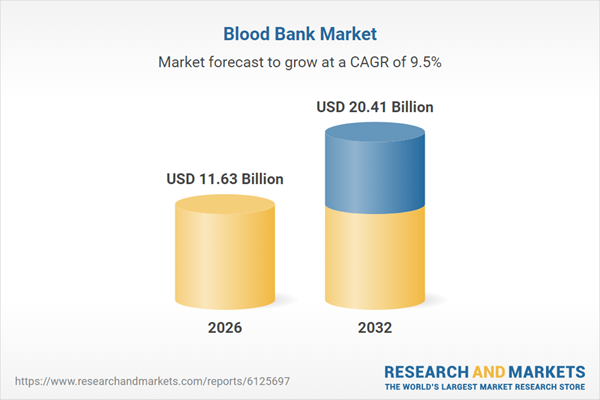
| Estimated Market Value ( USD | $ 11.63 Billion |
| Forecasted Market Value ( USD | $ 20.41 Billion |
| Compound Annual Growth Rate | 9.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |