Speak directly to the analyst to clarify any post sales queries you may have.
Food grade L-carnosine powder is moving from niche active to formulation-essential ingredient as wellness positioning and quality expectations intensify
Food grade L-carnosine powder is gaining renewed strategic relevance as brand owners and ingredient buyers recalibrate portfolios around clean-label positioning, everyday wellness, and targeted performance outcomes. As a naturally occurring dipeptide composed of beta-alanine and histidine and found in animal tissues, L-carnosine has long been recognized for its antioxidant activity and its role in buffering and anti-glycation pathways. In a food-grade context, the emphasis shifts from scientific curiosity to operational reality: consistent purity, controlled contaminants, documented traceability, and dependable sensory performance in real-world formulations.What makes this ingredient especially timely is the convergence of consumer demand for “do more with less” products and the industry’s push toward differentiated actives that can be integrated across multiple delivery formats. Functional foods, powders, capsules, tablets, and beverage systems all present distinct processing stresses, and L-carnosine’s performance is evaluated not only on label appeal but also on stability, compatibility, and manufacturability. Consequently, procurement teams increasingly scrutinize supplier qualification, method of manufacture, and quality documentation as closely as they evaluate the underlying bioactivity.
At the same time, the market’s competitive environment is being shaped by shifting trade policies, tighter quality expectations, and faster product cycles. Brands are seeking ingredients that can support premium positioning without exposing them to supply shocks or compliance surprises. This executive summary frames the landscape through the lens of practical decision-making-how the category is evolving, how tariffs are changing cost and sourcing strategies, how segmentation clarifies demand patterns, and how regional dynamics influence regulatory and commercial priorities.
Quality governance, format-first innovation, and resilience-focused procurement are redefining how food grade L-carnosine powder competes and scales
The landscape for food grade L-carnosine powder is undergoing a set of transformative shifts that are changing how value is created and captured across the ingredient supply chain. One of the most visible changes is the heightened expectation for end-to-end quality assurance. Buyers increasingly treat food-grade actives with the same rigor once reserved for pharmaceutical excipients, seeking comprehensive Certificates of Analysis, validated test methods, clear impurity profiles, and robust documentation that supports audits. This shift reflects not only regulatory diligence but also brand risk management as more products are sold through channels that demand tighter governance.In parallel, product development is being reshaped by a “format-first” mindset. Instead of beginning with an active and searching for an application, many innovation teams start with a delivery system-stick packs, ready-to-mix powders, gummies, capsules, or functional beverages-and then select actives based on solubility, taste masking requirements, hygroscopicity, stability under heat and pH stress, and compatibility with sweeteners, acids, and minerals. For L-carnosine powder, this means supplier support is increasingly evaluated in terms of technical service, application notes, and formulation troubleshooting, not simply price per kilogram.
Another meaningful shift is the growing preference for supply resilience. Procurement organizations are expanding qualifying supplier lists, seeking dual sourcing across geographies, and requesting more transparent disclosures on raw material origins and intermediate processing steps. The motivation is clear: logistics disruptions, geopolitical volatility, and changing tariff regimes can turn a seemingly stable sourcing strategy into a margin and continuity risk. As a result, supplier selection is becoming more strategic, with long-term agreements and contingency planning taking a more prominent role.
Finally, differentiation is being pursued through claims discipline and substantiation readiness. Even when an ingredient has well-known mechanisms, brand owners want claim language that aligns with local regulations and withstands scrutiny from retailers and platforms. This encourages tighter alignment between regulatory teams and marketing, as well as earlier engagement with suppliers on documentation packs, allergen statements, and compliance with food-safety frameworks. Together, these shifts are elevating L-carnosine powder from a commodity-like input to a managed strategic ingredient where trust, reproducibility, and technical collaboration drive purchasing decisions.
United States tariff dynamics in 2025 are compounding across landed cost, supplier diversification, and contract design for food grade L-carnosine powder
United States tariffs anticipated in 2025 introduce a cumulative impact that extends beyond simple price adjustments, influencing sourcing architecture, contract structures, and risk allocation across the value chain. For food grade L-carnosine powder, the most immediate implication is cost volatility tied to country-of-origin exposure and the classification pathways used at import. When tariffs rise or become more selectively applied, buyers typically respond by renegotiating Incoterms, building tariff-sharing clauses into supply agreements, and revisiting landed-cost models that include brokerage, duties, and compliance overhead.Over time, the tariff effect compounds through operational decisions. Importers may shift from opportunistic spot buying to more structured procurement, using longer-term contracts to stabilize costs and ensure supply continuity. This often increases the value of suppliers that can provide consistent documentation and predictable lead times, because any customs delay can magnify cost impacts through demurrage, production downtime, or missed promotional windows. As tariffs reshape trade flows, even suppliers outside the most affected corridors can experience demand spikes, tightening capacity and altering negotiation leverage.
Tariffs also tend to accelerate supplier diversification and nearshoring considerations, even when the ingredient itself remains globally traded. Some companies respond by qualifying alternative manufacturing sources, adjusting safety-stock policies, or moving certain finishing steps-such as blending, repacking, or final quality release-closer to the destination market. These steps can reduce exposure to some tariff structures while also improving responsiveness to customer requirements. However, they require rigorous change-control procedures to ensure that any shift in processing location does not compromise food-grade compliance or batch-to-batch consistency.
From a commercial perspective, the cumulative impact often shows up in pricing architecture and channel strategy. Brands may revisit pack sizes, subscription pricing, or promotional cadence to protect margins, while B2B suppliers may expand value-added services such as technical support, regulatory documentation, and inventory programs to justify pricing in a higher-cost environment. In this way, tariffs can function as a forcing mechanism that rewards operational maturity-companies with strong compliance systems, diversified sourcing, and disciplined cost-to-serve models are better positioned to maintain service levels and customer trust amid policy-driven turbulence.
Segmentation reveals distinct buying logic by application, channel expectations, and specification discipline that reshapes demand for food grade L-carnosine powder
Segmentation insights clarify where demand concentrates and why purchasing criteria differ so sharply across use cases for food grade L-carnosine powder. When viewed through product form and specification choices such as powder characteristics and purity expectations, buyers typically align requirements with the downstream process: tighter specifications and more extensive testing are favored for premium applications, while cost-sensitive programs may accept broader ranges provided compliance and performance remain reliable. This is also where sensory and handling considerations-flowability, dusting, and moisture management-start to influence supplier preference as much as assay values.Application-based segmentation further reveals that decision drivers vary by end-use context. In dietary supplement programs, the emphasis often sits on documentation readiness, batch consistency, and compatibility with common excipients, since capsules and tablets impose mechanical and stability constraints over shelf life. In functional foods and beverages, formulation realities can dominate, with developers focusing on taste interactions, pH behavior, and stability under thermal processing or high-shear mixing. Meanwhile, sports nutrition and active lifestyle products may prioritize performance positioning and rapid innovation cycles, which elevates the importance of agile supply and technical support for pilot-to-scale transitions.
Channel and customer-type segmentation also shapes how value is packaged. Brands selling through practitioner, specialty retail, and direct-to-consumer channels frequently require deeper technical narratives and stricter quality storytelling, while high-velocity ecommerce can reward consistent availability, standardized documentation packets, and operational reliability to avoid listing disruptions. In B2B ingredient supply relationships, procurement teams increasingly look for suppliers that can support audits, provide change notifications, and maintain robust traceability-capabilities that become differentiators when multiple sources appear similar on paper.
Finally, segmentation by packaging and order size underscores practical constraints that influence adoption. Smaller packs and flexible minimum order quantities can unlock innovation for emerging brands and contract manufacturers running multi-client lines, whereas large-volume buyers often optimize for cost, container utilization, and standardized packaging formats that reduce handling time. Across these segmentation lenses, the strongest opportunities tend to cluster where suppliers can align specification discipline, application support, and dependable fulfillment with the distinct operating model of each customer group. {{SEGMENTATION_LIST}}
Regional demand is shaped by compliance culture, climate and logistics realities, and channel sophistication across Americas, Europe, Middle East & Africa, and Asia-Pacific
Regional dynamics for food grade L-carnosine powder are defined by how regulatory culture, consumer preferences, and manufacturing ecosystems intersect. In the Americas, product positioning often emphasizes active wellness, performance, and premium quality cues, which raises expectations for documentation completeness and supply reliability. Buyers frequently seek strong audit readiness and clear labeling support, especially for products distributed through major retail and ecommerce platforms where compliance scrutiny can be intense.Across Europe, the operating environment tends to be shaped by rigorous quality management norms and careful claim governance. As a result, suppliers that can provide robust traceability, disciplined change control, and clear alignment with local food-safety expectations are advantaged. Product developers may move cautiously, prioritizing stability data and formulation predictability, particularly when integrating the ingredient into complex functional matrices. This can lengthen qualification cycles but can also support durable supplier relationships once approved.
In the Middle East and Africa, the market is often characterized by a mix of fast-growing wellness interest and varying regulatory maturity by country. This creates a practical need for suppliers and distributors that can navigate import documentation, ensure consistent quality, and support brand owners with compliant labeling and product registration workflows. As distribution networks diversify, reliability in logistics and packaging integrity becomes especially important in maintaining shelf stability under challenging climatic conditions.
Asia-Pacific presents a broad set of demand patterns, spanning advanced nutraceutical markets and high-capacity manufacturing hubs. Innovation cycles can be fast, and the region’s production and formulation ecosystems often enable rapid scaling when specifications are locked. At the same time, buyers may compare multiple sourcing options and place high value on consistency, transparent quality metrics, and responsive technical support. Across regions, commercial success increasingly depends on tailoring the supply model-documentation depth, packaging formats, and lead-time management-to the realities of each geography rather than applying a single global approach. {{GEOGRAPHY_REGION_LIST}}
Competitive advantage hinges on quality systems, technical formulation support, and cross-border compliance readiness among food grade L-carnosine powder suppliers
Key company insights in food grade L-carnosine powder center on how suppliers translate manufacturing capability into buyer confidence. Leading participants tend to differentiate through quality systems, including consistent batch documentation, validated analytical methods, and disciplined deviation and change-control practices. Because customers often evaluate multiple suppliers with similar assay claims, the deciding factors frequently become the reliability of Certificates of Analysis, the clarity of impurity and contaminant statements, and the ability to support audits without delays.Another competitive axis is technical enablement. Companies that provide formulation guidance, stability references, and practical troubleshooting for common dosage forms can reduce development time for customers and increase the likelihood of repeat business. This is particularly valuable where L-carnosine is incorporated into multi-ingredient blends, premixes, or acidic beverage systems that may challenge stability or sensory expectations. Suppliers that offer application labs, rapid sample turnaround, and responsive problem-solving often become preferred partners for brands that innovate quickly.
Operational execution also matters. Firms with resilient procurement of upstream inputs, redundant production or qualified subcontracting options, and mature inventory planning can maintain service levels during logistics shocks or policy-driven disruptions. In addition, companies that invest in packaging options, moisture control, and tamper-evident formats can better meet the needs of both large-volume industrial users and smaller brands seeking flexible ordering.
Finally, commercial strategy is increasingly tied to compliance readiness across borders. Companies that can provide region-specific documentation packs, clear statements on allergen handling and animal-origin considerations where relevant, and consistent labeling support are better positioned to serve global brands. In a category where trust and reproducibility are central, the companies that win are typically those that treat food grade L-carnosine powder as a high-governance ingredient and build customer relationships around transparency, technical competence, and dependable fulfillment.
Leaders can win with disciplined dual sourcing, formulation-to-procurement alignment, tariff-ready contracts, and trust-building compliance assets
Industry leaders can take practical steps now to strengthen competitiveness in food grade L-carnosine powder while reducing exposure to policy and supply volatility. First, prioritize supplier qualification as a living program rather than a one-time event. This means establishing clear acceptance criteria for documentation, audit responsiveness, impurity thresholds, and change notifications, and then reassessing suppliers on a defined cadence. As tariffs and logistics conditions evolve, maintaining at least one validated alternate source can protect continuity without sacrificing quality.Next, connect formulation strategy to procurement decisions earlier in the development cycle. When R&D teams share constraints related to pH, processing temperature, sensory targets, and shelf-life requirements, procurement can select specifications and packaging formats that reduce downstream rework. In practice, aligning on moisture management expectations, preferred particle characteristics, and compatibility with common excipients can prevent costly delays during scale-up and commercialization.
Leaders should also harden their tariff and trade playbooks. Incorporating scenario-based landed-cost modeling, building contract clauses that define duty responsibility, and pre-planning alternate routing or inventory buffers can reduce the operational shock when policy changes take effect. Where feasible, explore value-added steps closer to the end market-such as blending, repacking, or final release testing-provided change control and documentation remain robust.
Finally, invest in trust-building assets that shorten sales cycles. Standardized documentation packets, clear traceability narratives, and ready-to-use regulatory support reduce friction for customers and channel partners. Coupled with responsive technical service and predictable fulfillment, these actions position organizations to win long-term relationships in a market where buyers increasingly reward reliability as much as price or performance.
A triangulated methodology combining stakeholder interviews, structured secondary research, and validation loops to clarify decisions in L-carnosine sourcing
The research methodology underpinning this executive summary applies a structured, decision-oriented approach to understanding food grade L-carnosine powder across supply, demand, and operational risk. The work begins with structured secondary research to map the ingredient’s manufacturing pathways, common quality specifications used in food-grade trade, key application areas in supplements and functional foods, and the regulatory considerations that influence commercialization and labeling practices in major markets.This foundation is strengthened through primary inputs from industry participants across the value chain, including manufacturers, distributors, brand owners, formulators, and procurement or quality stakeholders. These conversations focus on real purchasing criteria, qualification timelines, documentation expectations, and the practical constraints encountered during formulation and scale-up. Qualitative insights are then normalized to identify recurring decision drivers, points of friction, and emerging requirements that differentiate suppliers.
To ensure coherence and usability, findings are triangulated across multiple evidence streams. Product and application trends are cross-checked against observed commercialization patterns, while trade and policy considerations are evaluated through an operational lens that includes landed-cost components, logistics constraints, and supplier diversification behaviors. Throughout the process, emphasis is placed on consistency, traceability of assumptions, and clear separation between verified practices and interpretive insights.
Finally, the research is organized into an executive-ready structure that links segmentation and regional dynamics to concrete implications for sourcing, product strategy, and risk management. The goal is to equip decision-makers with actionable clarity on how the market functions today and how to prepare for near-term disruptions without relying on speculative or unsupported claims.
The category is maturing into a trust-and-resilience market where quality governance, regional compliance, and application fit determine winners
Food grade L-carnosine powder is transitioning into a more governed, strategically managed ingredient category as wellness innovation, stricter quality expectations, and supply-chain volatility converge. Buyers are no longer evaluating suppliers solely on assay and price; they are assessing documentation depth, change control, traceability, and the ability to support real formulation and scale-up requirements across diverse formats.Transformative shifts in the landscape are rewarding companies that pair robust quality systems with technical enablement, while anticipated tariff dynamics in the United States for 2025 add urgency to resilient sourcing strategies and disciplined contract design. Segmentation clarifies that demand is not monolithic-application context, channel expectations, packaging needs, and specification discipline all materially change what “best supplier” means for a given buyer.
Regionally, differences in regulatory culture, distribution realities, and consumer preferences require tailored approaches rather than one-size-fits-all commercialization. Companies that invest in cross-border compliance readiness, operational resilience, and customer-facing trust assets will be better positioned to capture durable opportunities while managing risk in a more complex trading environment.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Food Grade L-Carnosine Powder Market
Companies Mentioned
The key companies profiled in this Food Grade L-Carnosine Powder market report include:- AIDP Inc
- Anhui Jinhe Bioengineering Co., Ltd.
- BLD Pharmatech Co., Ltd.
- ChromaDex Corp
- Hangzhou Kuan Biotechnology
- Hunan Nutramax Inc.
- Hunan Yangtze River Pharmaceutical Group
- Jiangyin Huachang Pharmaceutical Co., Ltd.
- Koninklijke DSM N.V.
- LactoSpore
- Lonza Group AG
- Merck KGaA
- Ningbo Zhenhai Haohai Chemical Co., Ltd.
- Nutracap Holdings
- Nutraceutical International Corporation
- Shandong Focuslong Demand Biotech Co., Ltd.
- Shanghai Freemen Chemical Technology Co., Ltd.
- Shanghai ShiYun Biotechnology Co., Ltd.
- Sino Biopharmaceutical Limited
- Suzhou Tianyu Biological Engineering Co., Ltd.
- Wuxi Duyuan Biotechnology
- Xi’an Realin Biotechnology Co., Ltd.
- Zhejiang LP Health Science Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
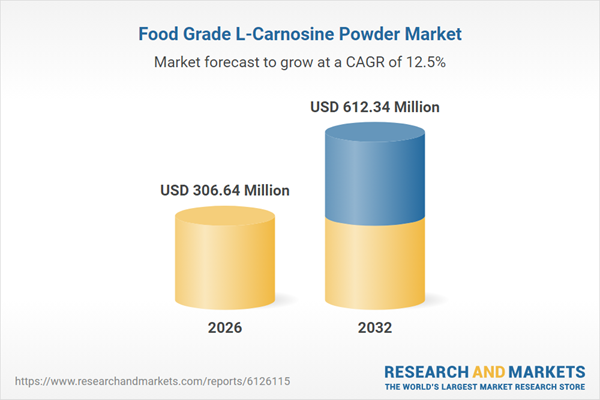
| Estimated Market Value ( USD | $ 306.64 Million |
| Forecasted Market Value ( USD | $ 612.34 Million |
| Compound Annual Growth Rate | 12.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |