Speak directly to the analyst to clarify any post sales queries you may have.
SGLT2 inhibitors are redefining cardio-renal-metabolic care, elevating access, adherence, and cross-specialty adoption as strategic priorities
SGLT2 inhibitors have moved from being primarily glucose-lowering agents to becoming foundational therapies spanning type 2 diabetes, heart failure, and chronic kidney disease. This broadening clinical utility has reshaped how prescribers think about cardio-renal-metabolic risk, placing outcomes such as hospitalization reduction and renal function preservation alongside glycemic control. As a result, the treatment landscape now rewards organizations that can align scientific messaging, real-world evidence, and patient access with evolving guideline pathways.At the same time, the category is being redefined by earlier intervention, more complex comorbidity profiles, and a heightened emphasis on long-term adherence. Clinicians increasingly look for therapies that reduce therapeutic burden and integrate well with background regimens, while payers scrutinize total cost of care across multi-year horizons. Consequently, success in SGLT2 inhibitors treatment is no longer determined solely by clinical differentiation; it depends equally on formulary positioning, patient affordability, and operational excellence across distribution channels.
Moreover, the market’s center of gravity is shifting toward integrated care models. Multidisciplinary teams involving endocrinologists, cardiologists, nephrologists, and primary care providers are expanding prescribing responsibility beyond a single specialty. This diffusion of decision-makers raises the bar for education, care pathway integration, and clear communication on safety considerations such as genital mycotic infections, volume depletion risk, and ketoacidosis precautions in select populations. In this context, stakeholders need an executive-level view that connects clinical momentum with commercial realities and policy-driven constraints.
From glucose control to integrated cardio-renal outcomes, the SGLT2 inhibitor landscape is shifting toward earlier use, pragmatic evidence, and value-based access
The landscape has undergone a transformative shift driven by a new consensus that metabolic, cardiovascular, and renal outcomes are inseparable in high-risk patients. As guideline bodies have broadened recommendations for SGLT2 inhibitors in heart failure and chronic kidney disease, therapy selection increasingly starts with risk phenotype rather than an A1C threshold alone. This reframing has made initiation more proactive, pushing treatment discussions upstream into primary care and specialty clinics where prevention of progression is central.In parallel, the evidence environment has become more pragmatic. Decision-makers are weighing randomized trial data alongside real-world evidence that clarifies persistence, discontinuation patterns, and outcomes in diverse populations, including older adults and patients with multiple comorbidities. Health systems are also prioritizing therapies that are easier to operationalize, with clearer monitoring protocols and fewer workflow disruptions, especially as clinician bandwidth tightens.
Another major shift is the rise of value-driven access design. Payers are increasingly sensitive to budget impact and are exploring tighter utilization management, step edits, and outcomes-aligned contracting approaches. This is occurring at the same time as patient affordability remains a persistent barrier, making copay support, patient assistance pathways, and simplified prior authorization processes a competitive differentiator.
Finally, competitive intensity is evolving beyond molecule-to-molecule comparisons. Brand trust, breadth of labeled indications, educational reach into cardiology and nephrology, and the ability to support population health programs have become central to positioning. Therefore, companies that treat SGLT2 inhibitors as a platform spanning multiple care pathways are better equipped to sustain growth than those approaching it as a single-indication diabetes segment.
Potential US tariffs in 2025 could reshape SGLT2 inhibitor supply economics, pushing resilience, sourcing diversification, and access continuity to the forefront
United States tariff dynamics anticipated for 2025 introduce a new layer of uncertainty for SGLT2 inhibitors treatment, particularly across pharmaceutical supply chains that rely on globally sourced active ingredients, intermediates, packaging components, and specialized manufacturing inputs. Even when finished-dose manufacturing occurs domestically, upstream dependencies can create exposure to cost variability and lead-time disruption. As tariff policies change, procurement teams may face higher input costs, prompting manufacturers and their partners to re-evaluate sourcing strategies and inventory buffers.The impact is likely to be uneven across the ecosystem. Companies with diversified supplier networks and flexible manufacturing footprints may absorb disruptions more effectively, while those reliant on concentrated sources could experience volatility in cost of goods and distribution planning. This can indirectly influence contracting strategies, channel availability, and the pace at which organizations can support expanded access initiatives. In parallel, wholesalers and specialty distributors may adjust purchasing behavior to manage risk, potentially affecting ordering patterns and short-term availability in certain channels.
Furthermore, tariffs can amplify broader policy and reputational considerations related to domestic manufacturing, supply security, and resilience. Health systems and payers are paying closer attention to drug availability and continuity of supply, particularly for therapies that have become embedded in chronic disease pathways. Consequently, manufacturers may invest more heavily in risk management, including dual sourcing, nearshoring of select inputs, and tighter coordination with contract development and manufacturing organizations.
Over time, these pressures may accelerate strategic moves toward supply-chain transparency and smarter demand planning. Organizations that model tariff scenarios, pre-negotiate supplier contingencies, and align commercial commitments with operational constraints will be better positioned to maintain consistent patient access, even as the policy environment becomes more unpredictable.
Segmentation reveals how drug type, indication, patient profile, channel strategy, and end-user setting now determine SGLT2 inhibitor adoption and persistence
Segmentation dynamics in SGLT2 inhibitors treatment increasingly reflect how clinical use cases are diversifying and how stakeholders prioritize outcomes beyond glycemic metrics. When viewed through segmentation by drug type, differentiation is less about class mechanism and more about the interplay between indication breadth, clinician familiarity, and the depth of supporting evidence across cardio-renal endpoints. This creates a competitive environment where brands with strong cross-specialty recognition can penetrate beyond traditional endocrinology settings.From the perspective of disease indication, the most consequential insight is that adoption is being driven by pathway ownership. Type 2 diabetes remains a core anchor, yet heart failure and chronic kidney disease are increasingly shaping prescribing behavior as cardiology and nephrology incorporate SGLT2 inhibitors into routine management. This multi-indication reality influences messaging, field deployment, and education strategies, because the same therapy may be positioned differently depending on whether the clinician’s primary goal is glycemic lowering, reducing hospitalization risk, or slowing renal decline.
Segmentation by patient population reveals the importance of tailoring initiation and monitoring approaches. Patients with multiple comorbidities, older adults, and those with variable renal function require nuanced counseling around hydration status, genitourinary adverse events, and sick-day management to mitigate rare but serious risks such as ketoacidosis in susceptible contexts. As these populations expand, successful programs emphasize patient education, care team alignment, and follow-up mechanisms that improve persistence.
Considering distribution channel segmentation, the route to the patient has become a strategic lever rather than a back-end function. Retail access supports scale, while specialty pharmacy engagement can improve navigation, benefits verification, and adherence support for complex plans. Meanwhile, hospital pharmacy dynamics matter as inpatient-to-outpatient transitions become a key moment for initiating therapy in heart failure and chronic kidney disease pathways. Finally, segmentation by end user underscores that prescribing is no longer concentrated in a single specialty; primary care, endocrinology, cardiology, and nephrology each influence uptake, requiring coordinated engagement and consistent, evidence-based communication across care settings.
Regional uptake varies with reimbursement design and care delivery maturity, making localized access, education, and pathway integration essential for SGLT2 inhibitors
Regional dynamics are shaped by differences in guideline adoption, payer architecture, care delivery models, and patient affordability, which together influence how quickly SGLT2 inhibitors become embedded in routine practice. In the Americas, strong integration of cardio-renal outcomes into clinical pathways is supporting broader use, yet access remains highly sensitive to formulary design, prior authorization burden, and copay affordability. As integrated delivery networks expand, there is increasing emphasis on standardized protocols that enable initiation across primary care and specialty clinics with consistent monitoring and education.Across Europe, the Middle East, and Africa, uptake reflects a complex mix of national reimbursement frameworks, health technology assessment approaches, and variation in specialist capacity. In markets with centralized decision-making, the strength of outcomes evidence and budget impact considerations heavily influence access conditions, which can shape the pace of adoption in heart failure and chronic kidney disease as well as diabetes. Meanwhile, differences in primary care infrastructure and specialist referral patterns can create uneven penetration within and across countries, making localized pathway engagement critical.
In Asia-Pacific, growth in chronic disease burden, evolving clinical guidelines, and expanding health coverage are important tailwinds, but affordability and access pathways vary widely by market. Urban centers with stronger specialist density may accelerate multi-indication use, while rural settings may rely more on primary care, requiring simplified initiation protocols and patient education that can scale. Additionally, distribution and dispensing practices can influence persistence, especially where follow-up frequency and medication continuity differ across care systems.
Taken together, the key regional insight is that winning strategies are those that adapt to local reimbursement realities and care delivery structures while maintaining consistent scientific positioning. Organizations that align stakeholder education, access design, and adherence support to regional constraints can improve continuity of therapy and strengthen real-world outcomes across diverse healthcare environments.
Competitive advantage increasingly comes from cross-specialty evidence, market access excellence, patient support depth, and resilient supply for SGLT2 inhibitors
Company performance in SGLT2 inhibitors treatment is increasingly determined by how effectively players extend relevance across diabetes, cardiology, and nephrology while maintaining trust on safety and tolerability. Leading organizations have invested in broad evidence generation, including outcomes-focused programs and real-world data initiatives that speak to payer and health system priorities. This enables more durable positioning because stakeholders can connect therapy use to downstream utilization outcomes and care pathway efficiency.Competitive differentiation also comes from commercial execution. Organizations with strong market access capabilities are better positioned to navigate utilization management, optimize formulary placement, and reduce friction in prescribing through hub services and benefits support. In addition, those with sophisticated provider education programs can engage multiple specialties with tailored materials that reflect each discipline’s priorities, from glycemic control parameters to heart failure hospitalization reduction and renal preservation.
Another defining capability is patient support design. Companies that offer clear initiation guidance, adherence tools, and side-effect management education can reduce discontinuation and strengthen persistence, particularly in patients who are new to the class or who have complex comorbidity profiles. Importantly, support models that coordinate with pharmacies and care teams can help bridge gaps during transitions of care, a moment when therapy continuation is often at risk.
Finally, manufacturing reliability and supply continuity are becoming more visible differentiators. As supply-chain scrutiny increases, companies that can demonstrate robust sourcing, quality management, and dependable distribution may earn greater confidence from health systems and payers. In a category increasingly seen as foundational to long-term chronic disease management, trust is built not only on clinical data but also on uninterrupted access and consistent patient experience.
Leaders can win by unifying cross-specialty pathways, simplifying access, investing in adherence, and hardening supply plans against policy volatility
Industry leaders should prioritize cross-specialty pathway ownership by aligning engagement across primary care, endocrinology, cardiology, and nephrology. This means translating evidence into discipline-specific value messages and practical initiation workflows, while ensuring consistent safety communication. When organizations make prescribing simple and predictable-especially around renal function considerations, diuretic adjustment awareness, and patient counseling-they reduce friction and encourage earlier adoption.Next, strengthen access strategy with an emphasis on reducing administrative burden. Streamlining prior authorization support, improving benefits verification turnaround, and partnering with pharmacies to minimize abandonment can materially improve starts and persistence. At the same time, leaders should prepare for more assertive payer management by building outcomes narratives that resonate with total cost of care discussions and by considering contracting approaches that reflect real-world utilization patterns.
In addition, invest in adherence and persistence as core value drivers. Practical education on hydration, genitourinary symptom recognition, and sick-day rules can prevent avoidable discontinuation and adverse events. Digital tools and care team prompts can reinforce follow-up, while transition-of-care programs can ensure continuity when patients move between inpatient and outpatient settings.
Finally, build tariff and supply volatility into strategic planning. Leaders should scenario-model policy-driven cost shocks, diversify critical suppliers where feasible, and coordinate commercial commitments with operational capacity. By connecting supply resilience with access continuity, companies can protect patient outcomes and maintain stakeholder confidence even during periods of policy uncertainty.
A triangulated methodology blending clinical, policy, and stakeholder inputs ensures reliable insights into SGLT2 inhibitor adoption, access, and real-world use
The research methodology integrates structured secondary research with targeted primary insights to develop a decision-oriented view of the SGLT2 inhibitors treatment environment. Secondary research draws on publicly available scientific literature, regulatory disclosures, clinical guideline updates, health policy documentation, and company communications to establish a foundational understanding of indications, safety considerations, and evolving standards of care.Primary research is designed to validate assumptions and capture current stakeholder priorities. Interviews and consultations are conducted with a cross-section of participants such as clinicians across relevant specialties, pharmacists, payer and health system stakeholders, and industry participants involved in market access, distribution, and patient support. These inputs help clarify real-world barriers to initiation, drivers of persistence, and the operational realities that influence adoption across care settings.
To ensure analytical rigor, insights are triangulated across multiple sources and checked for consistency. The analysis also applies segmentation and regional lenses to interpret how access, prescribing behavior, and care delivery models vary across settings. Throughout, the approach emphasizes transparency in reasoning, careful handling of conflicting signals, and a focus on actionable implications rather than speculative claims.
Finally, quality control is maintained through iterative reviews that test clarity, internal coherence, and relevance to decision-makers. This ensures the executive summary and supporting analysis remain grounded in current clinical practice and policy context while highlighting the strategic levers most likely to influence outcomes in SGLT2 inhibitors treatment.
SGLT2 inhibitors are becoming foundational across diabetes and cardio-renal care, making access design, adherence support, and supply resilience decisive factors
SGLT2 inhibitors treatment is now at the center of an integrated approach to managing chronic metabolic and cardio-renal disease, and the category’s trajectory is being shaped by forces that extend well beyond glucose control. As evidence and guidelines reinforce multi-indication utility, prescribing is expanding across specialties and moving earlier in the patient journey, raising the importance of education, workflow fit, and consistent safety communication.At the same time, access and affordability remain pivotal determinants of real-world impact. Payer management tools, administrative burden, and patient out-of-pocket exposure can slow adoption even when clinical rationale is strong. Therefore, organizations that pair scientific leadership with pragmatic access design and adherence support will be best positioned to sustain patient benefit.
Looking ahead, policy uncertainty such as potential tariff changes adds urgency to supply resilience and operational readiness. Companies that treat reliability of supply and continuity of access as strategic assets-alongside evidence generation and field execution-will be better equipped to support long-term therapy persistence and stakeholder confidence. In sum, success in this landscape requires integrated strategy across clinical, commercial, and operational dimensions.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China SGLT2 Inhibitors Treatment Market
Companies Mentioned
The key companies profiled in this SGLT2 Inhibitors Treatment market report include:- AstraZeneca PLC
- Boehringer Ingelheim International GmbH
- Cipla Limited
- Dr. Reddy’s Laboratories Limited
- Eli Lilly and Company
- Johnson & Johnson
- Merck & Co., Inc.
- Mylan N.V.
- Pfizer Inc.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
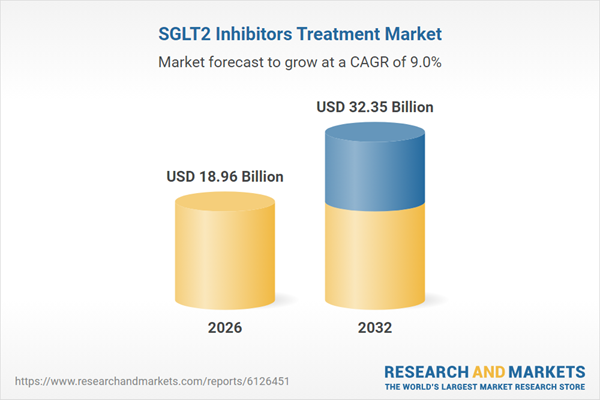
| Estimated Market Value ( USD | $ 18.96 Billion |
| Forecasted Market Value ( USD | $ 32.35 Billion |
| Compound Annual Growth Rate | 9.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |