Speak directly to the analyst to clarify any post sales queries you may have.
Setting the context for N-acetylglutamate synthetase deficiency as diagnostics, acute care urgency, and rare-disease infrastructure converge
N-acetylglutamate synthetase deficiency is a rare urea cycle disorder characterized by impaired activation of carbamoyl phosphate synthetase I, leading to hyperammonemia that can present catastrophically in neonates or episodically in later-onset patients. Because the disorder is uncommon and symptoms overlap with other metabolic and neurologic conditions, the route from first clinical signs to definitive diagnosis is often complex, time-sensitive, and operationally demanding for care teams.In parallel, the landscape supporting patients and providers has been changing. Advances in genomic diagnostics, improvements in metabolic intensive care protocols, and broader awareness of hyperammonemia as a medical emergency are pushing systems toward earlier recognition and faster intervention. At the same time, disparities in access to specialized centers, variability in newborn screening policies, and constraints on rare-disease supply chains continue to influence how consistently patients receive standard-of-care evaluation and treatment.
This executive summary synthesizes the forces shaping the N-acetylglutamate synthetase deficiency ecosystem across diagnostics, therapeutics, care delivery, and enabling infrastructure. It emphasizes how stakeholders are modernizing pathways from suspicion to confirmation, aligning acute and long-term management, and building resilience into sourcing and distribution, all while navigating evolving policy and reimbursement expectations.
How care pathways are moving from isolated specialist practice to integrated, faster diagnosis and networked management for hyperammonemia risk
The market environment around N-acetylglutamate synthetase deficiency is shifting from isolated, center-dependent management toward more connected care pathways that prioritize speed, standardization, and longitudinal follow-up. One transformative shift is the growing expectation that hyperammonemia triggers a defined emergency algorithm, supported by readily available ammonia testing, rapid confirmatory workflows, and immediate metabolic consultation. This operational emphasis is changing procurement behavior, training priorities, and the types of solutions hospitals seek for both acute stabilization and downstream evaluation.Another shift is the normalization of genomic testing within neonatal and pediatric diagnostic routines. Wider availability of next-generation sequencing, paired with improved interpretation frameworks and curated rare-disease knowledgebases, is shortening time-to-diagnosis for many suspected urea cycle disorders. Importantly, this does not eliminate the need for biochemical confirmation; instead, it elevates the value of integrated workflows where plasma amino acids, urine orotic acid, and molecular testing inform each other rather than compete for primacy.
Care delivery is also evolving through more formalized networks linking tertiary metabolic centers with community hospitals. Telehealth-enabled consultation, shared care plans, and standardized transfer criteria are increasingly used to reduce delays in initiating ammonia-lowering interventions and to support safer transitions back to local care. As a result, stakeholders are paying closer attention to education for emergency physicians and intensivists, as well as to the availability of protocols that translate specialist knowledge into actionable bedside steps.
Finally, rare-disease development itself is being reshaped by platform thinking. Stakeholders are leveraging learnings across urea cycle disorders, exploring real-world evidence strategies, and building patient registries that can support both clinical research and quality improvement. This shift matters in N-acetylglutamate synthetase deficiency because patient numbers are limited; therefore, evidence generation increasingly depends on multi-center collaboration, harmonized data capture, and pragmatic endpoints that reflect clinically meaningful outcomes such as avoidance of hyperammonemic crises and preservation of neurodevelopmental function.
Why United States tariff dynamics in 2025 could reshape sourcing resilience, lead times, and access continuity across rare metabolic care pathways
United States tariff changes anticipated in 2025 create a set of operational pressures that can be disproportionately felt in rare metabolic disorders, where supply chains are narrow, redundancy is limited, and continuity of access is clinically critical. Even when finished pharmaceuticals are protected through established trade and regulatory mechanisms, upstream dependencies such as active pharmaceutical ingredients, key intermediates, specialized excipients, and single-use bioprocessing components can face cost volatility and lead-time risk if sourced internationally.For N-acetylglutamate synthetase deficiency, the impact is most pronounced in the reliability of supportive-care inputs and the broader ecosystem that enables timely treatment initiation. Hospitals and specialty pharmacies may encounter higher landed costs or longer replenishment cycles for select imported components used in compounding, cold-chain packaging, diagnostic consumables, and emergency department laboratory supplies. Consequently, procurement teams are expected to shift from price-first purchasing to resilience-first contracting, including dual sourcing, longer-term agreements, and stronger supplier quality documentation.
Tariffs can also influence clinical trial and compassionate-use logistics. Sponsors running small, multi-site programs may see increased complexity in moving investigational materials, reference standards, and specialized testing kits across borders, especially when compliance and customs clearance processes add friction. In rare disease contexts, these incremental delays can have outsized implications for enrollment timelines and protocol adherence.
In response, many stakeholders are preparing through localization strategies and scenario planning. These include qualifying alternate suppliers, increasing safety-stock for critical inputs, and simplifying packaging configurations to reduce exposure to tariff-classified materials where feasible. Over time, the cumulative effect is likely to push more organizations toward end-to-end visibility tools that connect demand sensing at metabolic centers to manufacturing and distribution decisions, thereby reducing the probability that trade-related disruptions translate into clinical access gaps.
Segmentation insights that explain how diagnosis pathways, care setting roles, and patient age profiles shape adoption across the full care continuum
Segmentation reveals that demand patterns are driven less by broad volume and more by urgency, confirmatory confidence, and continuity of long-term management. When viewed by diagnosis type, clinical behavior diverges between newborn screening follow-up and symptom-driven workups, with the former emphasizing rapid confirmatory testing and standardized reflex pathways, while the latter often requires broader differential diagnosis across neurologic and hepatic presentations. This difference influences which clinical settings prioritize rapid biochemical testing versus expanded molecular panels and how quickly patients are escalated to specialist care.Looking through the lens of treatment type, the ecosystem spans acute crisis stabilization, ammonia-scavenging and supportive measures, and longer-term metabolic management with targeted therapy where clinically appropriate. Acute-care decision-making is often protocolized and time-bound, while chronic management is more individualized, depending on phenotype severity, neurodevelopmental trajectory, and adherence feasibility. As a result, stakeholders increasingly value solutions that reduce variability, such as standardized order sets, integrated monitoring, and coordinated access through specialty pharmacy services.
By age group, the needs of neonatal-onset patients differ materially from pediatric or adult presentations. Neonates require rapid escalation and intensive monitoring, with care teams focused on preventing neurologic injury during the first crisis. Later-onset patients, including adolescents and adults, more often need systems that prevent recurrence, support life transitions, and manage comorbidities, including pregnancy considerations and intercurrent illness planning. This age-related segmentation also shapes education priorities, as adult emergency settings may be less familiar with urea cycle disorders and require explicit hyperammonemia triggers.
Considering end user segmentation, tertiary metabolic centers act as anchors for diagnosis confirmation and care planning, but community hospitals, emergency departments, and outpatient specialty clinics increasingly influence time-to-treatment. Specialty pharmacies and home-infusion or home-care providers also become central in maintaining continuity, particularly where long-term therapies and medical nutrition support require reliable delivery. Finally, when assessed by distribution channel, controlled, high-touch pathways-often routed through specialty distribution-tend to dominate, reflecting the need for cold-chain integrity, prior authorization navigation, and patient support services that go beyond standard wholesaler models.
Regional insights showing how screening maturity, referral networks, and health-system readiness determine diagnostic speed and therapy continuity worldwide
Regional dynamics in N-acetylglutamate synthetase deficiency are shaped by how consistently hyperammonemia is recognized, how newborn screening and confirmatory testing are organized, and whether referral networks can move patients rapidly to specialist oversight. In the Americas, mature emergency care infrastructure and established metabolic centers support rapid intervention, yet variability across states and provinces in screening policies and specialist density can create uneven time-to-diagnosis and follow-up intensity. Cross-institutional protocols and shared care models are increasingly used to reduce that variability and to support community hospitals managing first presentations.Across Europe, the Middle East & Africa, heterogeneity is even more pronounced. Several European countries benefit from structured rare-disease networks and reimbursement frameworks that can support specialty access, while cross-border care coordination remains an important mechanism for ultra-rare conditions. In parts of the Middle East, investments in tertiary hospitals and genomic programs are strengthening diagnostic capability, but supply-chain resilience and standardized long-term follow-up can differ widely by country. In many African settings, limited access to metabolic testing and specialized nutrition support can delay diagnosis and complicate ongoing management, elevating the importance of regional centers of excellence and targeted capacity building.
In Asia-Pacific, rapid expansion of genetic testing capacity and growing awareness of inborn errors of metabolism are improving identification, particularly in high-resource markets with strong pediatric specialty networks. However, fragmentation in healthcare delivery and disparities between urban and rural regions can still impede consistent emergency response and continuity of care. As screening programs broaden and digital health tools mature, the region is positioned for more systematic pathways, but stakeholders must balance innovation with practical considerations such as cold-chain distribution, trained metabolic dietitians, and consistent access to confirmatory biochemical assays.
Company insights across therapy developers, diagnostics, medical nutrition, and specialty distribution shaping end-to-end patient access and continuity
Company activity in this space is best understood through the roles organizations play across the rare-disease value chain rather than through sheer portfolio size. Branded pharmaceutical developers and specialty rare-disease companies focus on maintaining reliable access to targeted therapies and on expanding clinician education to reduce missed hyperammonemia cases. Their differentiation increasingly depends on evidence packages that demonstrate real-world utility, patient support programs that reduce administrative friction, and distribution models designed for time-sensitive initiation.Diagnostic companies and laboratory service providers are equally influential. Innovations in rapid sequencing, improved variant interpretation, and reflex testing workflows are enabling faster triage of suspected urea cycle disorders. Laboratories that can integrate biochemical and molecular data, provide clear interpretive reports, and offer clinician-facing consultation are becoming key partners for hospitals that must make urgent decisions in the absence of complete information.
Medical nutrition and metabolic support providers also shape outcomes by ensuring that nutritional therapy, specialized formulas, and care coordination are available and adaptable across life stages. In practice, these organizations must operate with high service intensity, helping families navigate adherence, managing supply continuity, and coordinating with clinicians during intercurrent illnesses. Finally, distributors and specialty pharmacies differentiate on cold-chain reliability, benefits verification expertise, and the ability to maintain consistent patient engagement, all of which matter when therapy interruptions can translate into preventable clinical deterioration.
Action-ready recommendations to strengthen emergency readiness, evidence generation, supply resilience, and patient experience in ultra-rare care
Industry leaders can improve impact by prioritizing speed-to-action in hyperammonemia pathways. This begins with operationalizing standardized emergency department protocols, ensuring ammonia testing is available and trusted, and embedding reflex steps that quickly connect clinicians with metabolic specialists. Building these pathways in collaboration with hospitals reduces variability and supports earlier stabilization, which is central to protecting neurologic outcomes.Next, organizations should invest in integrated evidence generation that fits the realities of ultra-rare disease. Multi-center registries, harmonized data definitions, and pragmatic outcome measures can strengthen both clinical credibility and payer conversations without relying on overly burdensome trial designs. Where possible, aligning data capture with routine care workflows reduces site fatigue and improves longitudinal completeness.
Supply resilience should be treated as a strategic differentiator. Leaders can map tariff and logistics exposure across APIs, excipients, packaging, and diagnostics consumables, then execute dual-sourcing and safety-stock strategies for truly critical inputs. This approach should be paired with quality-by-design and supplier qualification programs that prevent last-minute substitutions from creating regulatory or clinical risk.
Finally, patient and provider experience must be designed intentionally. Streamlining prior authorization, providing case management that supports transitions from neonatal intensive care to outpatient life, and equipping families with clear sick-day plans can reduce preventable crises. Education initiatives that target non-specialist clinicians-especially emergency and adult-care teams-can further close gaps where late-onset presentations are most likely to be missed or mismanaged.
Methodology built for ultra-rare disorders, combining clinical, operational, and policy inputs to validate real-world pathways and constraints
The research methodology integrates qualitative and desk-based approaches tailored to an ultra-rare disorder where insight density matters more than broad sampling. The process begins with structured secondary research across peer-reviewed literature, clinical guideline publications, regulatory communications, and publicly available information from health systems and patient organizations. This establishes a baseline view of disease burden, diagnostic practices, treatment paradigms, and evolving standards of care.Building on that foundation, the methodology incorporates targeted primary inputs from stakeholders involved in diagnosis, acute management, long-term follow-up, and access facilitation. This includes clinician perspectives from metabolic specialists and critical care teams, as well as operational viewpoints from pharmacy, distribution, and care coordination functions. The aim is to triangulate how decisions are made in real settings, where constraints such as laboratory turnaround time, referral friction, and reimbursement administration shape outcomes.
Analysis emphasizes consistency checks across sources, reconciliation of divergent viewpoints, and careful separation of established practice from emerging experimentation. The approach also includes mapping of value-chain dependencies, with attention to where supply, policy, and logistics can disrupt continuity. Throughout, findings are synthesized into actionable themes that help decision-makers prioritize investments in diagnostics integration, protocol deployment, and resilient access models without relying on speculative sizing exercises.
Bringing the narrative together as faster diagnosis, resilient access, and standardized crisis response become the real differentiators in care
N-acetylglutamate synthetase deficiency sits at the intersection of medical urgency and system readiness. While the condition is rare, the operational requirements it imposes-rapid recognition of hyperammonemia, immediate initiation of stabilization protocols, and coordinated long-term follow-up-are increasingly viewed as benchmarks for high-functioning metabolic care. Consequently, organizations that can reduce time-to-diagnosis and standardize emergency response are positioned to deliver meaningful clinical value.At the same time, the environment is becoming more complex. Genomic integration, networked care models, and patient-support expectations are rising, while trade and sourcing pressures introduce new fragility into already narrow supply chains. The most successful stakeholders will be those that treat access continuity, evidence generation, and provider enablement as a unified strategy rather than separate initiatives.
Ultimately, progress in this space will be measured by fewer missed or delayed diagnoses, smoother transitions across care settings, and fewer preventable hyperammonemic crises. Aligning clinical protocols, diagnostic workflows, and resilient distribution models provides a practical path to that outcome, even in a disorder where patient numbers remain limited.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China N-Acetylglutamate Synthetase Deficiency Market
Companies Mentioned
The key companies profiled in this N-Acetylglutamate Synthetase Deficiency market report include:- AmmoniaCare Inc.
- Ascendis Pharma A/S
- Bayer AG
- BioMarin Pharmaceutical Inc.
- Hoffmann-La Roche Ltd.
- Horizon Therapeutics plc
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Sarepta Therapeutics, Inc.
- Sobi
- Takeda Pharmaceutical Company Limited
- Ultragenyx Pharmaceutical Inc.
- Vertex Pharmaceuticals Incorporated
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
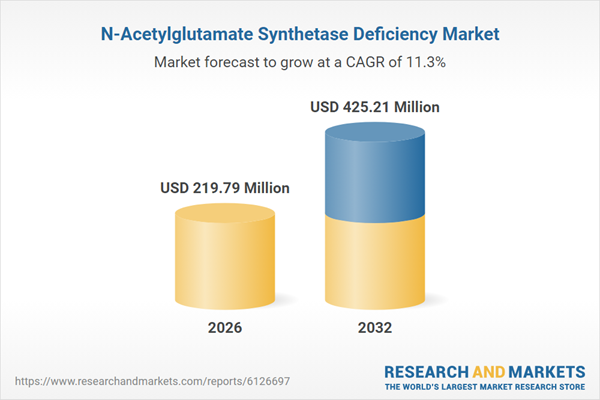
| Estimated Market Value ( USD | $ 219.79 Million |
| Forecasted Market Value ( USD | $ 425.21 Million |
| Compound Annual Growth Rate | 11.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |