Speak directly to the analyst to clarify any post sales queries you may have.
Diabetic lancets are shifting from a basic consumable to a patient-experience and supply-resilience priority in modern diabetes care
Diabetic lancets remain a foundational, high-frequency component of self-monitoring and clinical glucose testing, even as diabetes management becomes more digital and outcomes-driven. Their apparent simplicity hides a complex set of priorities for manufacturers, providers, payers, and distributors: consistent puncture performance, minimal pain, reliable sterility, and compatibility with lancing devices and workflows. As a result, the category is evolving from a commoditized consumable toward a quality- and experience-led product choice that affects adherence, patient satisfaction, and operational efficiency.At the same time, demand patterns are shaped by the growing prevalence of diabetes, a greater emphasis on patient-centric care, and the expansion of home-based testing supported by remote care models. Purchasers increasingly weigh total cost of use rather than unit price alone, considering factors such as packaging efficiency, training burden, sharps disposal practices, and the stability of supply. In this context, diabetic lancets occupy a strategic position at the intersection of preventive care, chronic disease management, and supply chain resilience.
This executive summary synthesizes the major shifts influencing diabetic lancets today, highlights tariff-related considerations in the United States for 2025, and frames what matters most across product and customer segments, regions, and leading companies. The intent is to provide decision-makers with a practical lens for prioritizing investment, mitigating risk, and capturing value in a category where trust and reliability drive repeat utilization.
Comfort, safety engineering, digital-adjacent workflows, and supply resilience are redefining competitive advantage in diabetic lancets
The landscape for diabetic lancets is being reshaped by a convergence of patient expectations, care delivery redesign, and manufacturing and regulatory modernization. A primary shift is the elevation of comfort and ease-of-use from “nice to have” to a differentiator that affects adherence. Users are more aware of needle gauge options, penetration depth settings, and the relationship between lancet sharpness and perceived pain, and they increasingly expect consistent performance across high-volume daily use.Alongside comfort, safety engineering has become more central. Healthcare facilities and home caregivers are pushing for designs that reduce accidental sticks and simplify compliant disposal, reinforcing momentum toward safety lancets in professional environments. This is reinforced by ongoing occupational safety standards and facility-level protocols that favor single-use, integrated safety mechanisms. In parallel, sustainability and waste reduction pressures are beginning to influence packaging, logistics, and material selection, particularly where large institutions track environmental performance.
Another transformative change is the tightening integration of consumables into broader diabetes ecosystems. Even though lancets are not digital devices, they sit within workflows that are increasingly digitized. Procurement teams value compatibility with device portfolios, predictable replenishment, and vendor reliability that supports subscription-like supply models. Retail and e-commerce channels continue to expand, which increases price transparency while also rewarding brands that communicate quality clearly and meet rapid fulfillment expectations.
Finally, manufacturing strategies are shifting toward resilience. Companies are diversifying supplier bases, reassessing tooling and automation, and prioritizing quality systems that reduce variability. Regulatory expectations for traceability, labeling precision, and post-market surveillance continue to raise the bar, especially for firms supplying large tenders and institutional contracts. Taken together, these shifts are moving the category toward fewer tolerated failures, faster switching when performance disappoints, and stronger preference for partners that can deliver continuity through disruptions.
United States tariffs in 2025 are amplifying cost volatility and accelerating sourcing, contracting, and product-mix decisions for lancets
United States tariff dynamics in 2025 introduce a material planning variable for diabetic lancets because the category relies on globalized sourcing for needles, plastics, packaging components, and finished-device assemblies. When tariff exposure increases or becomes more uncertain, landed cost volatility can ripple through tenders, retail pricing, and distributor margins. The immediate impact is rarely uniform: it differs by country-of-origin footprint, the proportion of value added in each manufacturing step, and how contracts are structured across public and private buyers.In response, many suppliers are expected to intensify “tariff engineering” and cost-to-serve optimization. That can include shifting final assembly locations, reclassifying components where legitimately applicable, increasing domestic finishing steps, or renegotiating supplier terms to share cost burdens. However, these adjustments require rigorous compliance, documentation, and quality validation. For regulated medical products, even seemingly small manufacturing changes can trigger requalification work, updated labeling, and additional oversight, which can compress timelines and add indirect cost.
Procurement organizations are also likely to react by shortening pricing windows, adding tariff pass-through clauses, or diversifying awarded vendors to hedge supply and cost shocks. This can be advantageous for manufacturers with redundant capacity and transparent documentation, but it challenges smaller players with limited sourcing options. Distributors may adjust inventory buffers to manage timing risk around tariff implementation, which can temporarily influence availability and lead times.
Over the medium term, tariffs can accelerate structural shifts already underway. Suppliers may invest more aggressively in automation to preserve margin while maintaining quality. Some may prioritize higher-value segments such as safety lancets and differentiated gauges where pricing is less purely commodity-driven. The net effect is that tariff pressure in 2025 is not only a cost story; it is a catalyst for rethinking manufacturing geography, contract strategy, and product mix to maintain stability and competitiveness.
Segmentation reveals that lancet success hinges on product design, gauge credibility, care setting workflows, and channel-specific purchasing behavior
Segmentation insights for diabetic lancets increasingly reflect how purchasing decisions are made in practice: by product design expectations, end-user setting, distribution pathway, and fit with prevailing care routines. When viewed through product type, safety-oriented designs tend to align with clinical protocols that emphasize needlestick prevention and simplified disposal, while standard lancets remain prevalent where cost sensitivity and device familiarity dominate. This distinction is not merely technical; it shapes training requirements, incident risk management, and the operational acceptance of new vendors.From the perspective of gauge and needle geometry, finer gauges are typically associated with comfort-focused positioning, yet real-world satisfaction depends on consistency, sharpness retention during manufacturing, and how well the lancet pairs with common lancing devices. Buyers increasingly look for proof of quality control rather than relying on gauge claims alone. Depth adjustability in the lancing device ecosystem further complicates this, making compatibility and user education influential factors in product selection.
End-use segmentation reveals an important divergence between home care and institutional environments. Home users prioritize comfort, affordability over time, and easy replenishment through pharmacies and online channels, while hospitals, clinics, and diagnostic settings prioritize safety protocols, standardization, and throughput efficiency. In long-term care settings, staff workflows and compliance auditing make reliability, packaging practicality, and sharps management especially critical, often favoring solutions that reduce handling steps.
Distribution-channel segmentation highlights a growing split between traditional medical supply routes and consumerized purchasing. Retail pharmacies remain central for many users, but e-commerce expansion has strengthened direct-to-consumer availability and intensified brand competition. Meanwhile, group purchasing and distributor-led contracting continue to shape institutional demand, where tender requirements can include documentation depth, manufacturing certifications, and stable supply assurances. Across these segmentation lenses, the most successful strategies connect product attributes to the user’s daily routine, minimizing friction while meeting the safety and compliance expectations of each setting.
Regional realities across the Americas, Europe Middle East & Africa, and Asia-Pacific determine channel power, compliance demands, and value expectations
Regional dynamics in diabetic lancets are shaped by differences in diabetes care infrastructure, reimbursement patterns, regulatory frameworks, and channel maturity. In the Americas, purchasing behavior often blends institutional standardization with strong retail and e-commerce demand for home testing supplies. Buyers in this region place high value on dependable supply, consistent quality, and transparent product specifications, while competitive intensity encourages vendors to differentiate on comfort claims, safety features, and channel partnerships.Across Europe, Middle East & Africa, the market environment is more heterogeneous, reflecting diverse reimbursement systems and procurement models. Many European countries emphasize compliance, tender documentation, and standardized product performance, which can elevate quality certifications and traceability. In parts of the Middle East, investment in healthcare capacity and modernized procurement can create openings for premium, safety-forward offerings, while certain African markets may face affordability constraints and logistics challenges that increase the importance of distributor reach and resilient inventory planning.
In Asia-Pacific, a combination of large diabetic populations, expanding access to diagnostics, and rapidly developing retail and digital commerce ecosystems supports broad adoption across multiple price tiers. Local manufacturing capabilities and policy priorities can influence sourcing strategies and competitive positioning, particularly where governments seek to strengthen domestic medical supply chains. In parallel, urban consumers in several Asia-Pacific markets demonstrate strong receptivity to comfort and convenience messaging, while rural access considerations heighten the need for efficient distribution and pragmatic packaging.
Across all regions, decision-makers increasingly evaluate how suppliers manage regulatory updates, quality systems, and supply continuity. As cross-border trade conditions evolve, regional procurement teams are paying closer attention to country-of-origin risk, lead-time variability, and the ability to sustain service levels during disruption. Vendors that tailor channel strategy and compliance support to each regional reality are more likely to earn repeat contracts and long-term loyalty.
Competitive advantage is built on quality discipline, safety and comfort differentiation, channel partnerships, and resilient manufacturing footprints
Key companies in diabetic lancets compete in a category where trust is built through repeat performance, rigorous quality systems, and dependable fulfillment. Established medical device manufacturers often leverage broad diabetes portfolios and long-standing relationships with providers and distributors, using reliability and compliance documentation as core advantages. Their strategies frequently emphasize consistent manufacturing, strong packaging and labeling discipline, and compatibility with widely used lancing devices.Consumer-health brands and retail-anchored suppliers compete by translating clinical credibility into clear, accessible messaging that resonates with home users. These companies tend to focus on comfort cues such as perceived pain reduction, ease of handling, and simple replenishment through omnichannel distribution. Private-label and value-focused players, by contrast, compete on cost efficiency and channel leverage, particularly in high-volume retail settings, but they face greater scrutiny as buyers increasingly demand proof of quality consistency.
Across the competitive set, differentiation is increasingly driven by safety innovation, ergonomic design, and operational support for institutional accounts. Companies that can provide education materials, clear instructions for use, and packaging formats optimized for clinical workflows can reduce adoption friction. Additionally, firms with diversified manufacturing footprints and strong supplier relationships are better positioned to manage tariff-related volatility and reduce disruption risk.
Partnerships are also becoming more prominent. Manufacturers collaborate with distributors, pharmacy chains, and device ecosystem partners to secure shelf space, align replenishment programs, and improve availability. In this environment, company success depends less on novelty and more on disciplined execution: quality assurance, regulatory readiness, supply reliability, and a product story that aligns with how patients and clinicians actually use lancets day after day.
Leaders can win through comfort credibility, tariff-ready sourcing, channel-specific execution, and safety-plus-sustainability operational improvements
Industry leaders can strengthen their position by treating lancets as an adherence and workflow tool rather than a low-involvement commodity. Prioritizing consistent puncture performance and validated sharpness metrics can reduce variability that drives user dissatisfaction and brand switching. Where comfort claims are central, leaders should pair messaging with credible quality controls, clear compatibility guidance, and education that helps users select gauge and depth settings appropriately.To manage 2025 tariff uncertainty, leaders should stress-test country-of-origin exposure and map alternative sourcing pathways before disruptions occur. This includes dual-qualifying critical inputs, clarifying contract language around tariff pass-through, and aligning inventory strategy with lead-time risk. When manufacturing changes are needed, early coordination with regulatory and quality teams can prevent rework and avoid compliance delays that offset intended savings.
Commercially, leaders should align channel strategy with the realities of purchasing behavior in home and institutional segments. In retail and e-commerce, improving findability, packaging clarity, and replenishment convenience can translate directly into repeat purchases. In institutional settings, simplifying standardization through documentation packets, training assets, and packaging designed for high-throughput use can improve win rates and renewal likelihood.
Finally, leaders should invest in sustainability and safety where it can be operationalized without compromising sterility and compliance. Even incremental improvements in packaging efficiency, waste reduction, and sharps handling can resonate with health systems under environmental and safety mandates. Over time, companies that combine patient-centric design with procurement-ready execution will be best positioned to earn durable demand across shifting policy and supply conditions.
A triangulated methodology combining stakeholder input, regulatory and product validation, and structured competitive assessment supports reliable insights
The research methodology for this report blends structured primary engagement with rigorous secondary review to build a decision-oriented view of the diabetic lancets landscape. Primary inputs include interviews and consultations with stakeholders across the value chain, such as manufacturers, distributors, procurement professionals, clinicians, and other informed participants who can speak to product requirements, purchasing criteria, and workflow realities. These conversations are used to validate assumptions, identify adoption barriers, and clarify how competitive differentiation is perceived in day-to-day use.Secondary research draws on publicly available and authoritative materials such as regulatory and standards documentation, company filings and product literature, trade and customs information where applicable, and credible publications relevant to diabetes care and medical consumables. This step is designed to triangulate claims, confirm product positioning, and understand changes in compliance expectations, manufacturing practices, and channel evolution.
Analytical work includes segment mapping, qualitative competitive assessment, and consistency checks across inputs to reduce bias and improve reliability. Attention is given to aligning terminology across regions and channels, particularly where similar products are marketed differently or where labeling conventions vary. The methodology emphasizes transparency in how insights are derived, prioritizing actionable interpretation over speculative conclusions.
Throughout the process, findings are reviewed for internal coherence and practical relevance to decision-makers, ensuring that the final narrative connects product attributes and procurement behavior to the broader shifts affecting diabetes care delivery and supply chain stability.
Rising expectations and tariff-linked uncertainty are pushing diabetic lancets toward higher standards of execution, resilience, and user-centered design
Diabetic lancets are experiencing meaningful change despite their long-established role in glucose monitoring. Comfort and safety expectations are rising, purchasing channels are becoming more consumerized, and institutional buyers are tightening requirements around compliance, standardization, and reliability. These forces are elevating the importance of quality systems, documentation discipline, and consistent user experience, pushing the category toward more deliberate product and supplier choices.Tariff-related uncertainty in the United States in 2025 adds another layer of complexity, encouraging suppliers and buyers to revisit sourcing footprints, contract design, and inventory strategies. Rather than treating tariffs as a short-term disruption, leading organizations are using them as a prompt to build resilience through diversification, automation, and a sharper focus on differentiated products where value can be sustained.
Across segments and regions, the common thread is execution. Companies that align product design with real workflows, support channels with clear communication, and maintain dependable supply are more likely to earn repeat adoption. As diabetes care continues to evolve, lancets will remain a daily-use touchpoint where small improvements in performance, safety, and accessibility can produce outsized trust and loyalty over time.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Diabetic Lancets Market
Companies Mentioned
The key companies profiled in this Diabetic Lancets market report include:- Abbott Laboratories
- Arkray, Inc.
- Ascensia Diabetes Care Holdings AG
- B. Braun Melsungen AG
- F. Hoffmann-La Roche Ltd
- LifeScan, Inc
- Medtronic plc
- Narang Medical Limited
- Owen Mumford Ltd
- Sterimed Group
- Trividia Health, Inc.
- Ypsomed AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
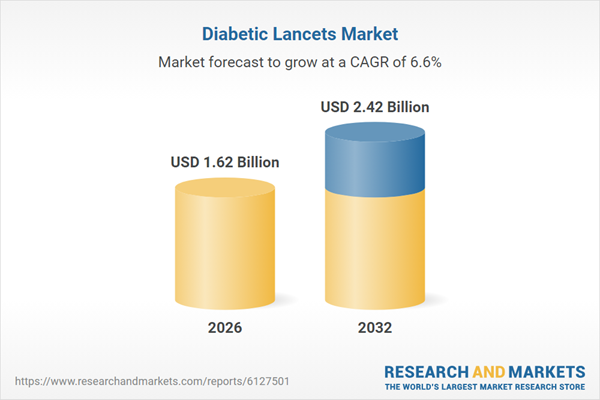
| Estimated Market Value ( USD | $ 1.62 Billion |
| Forecasted Market Value ( USD | $ 2.42 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |