Speak directly to the analyst to clarify any post sales queries you may have.
Dry eye software is evolving into a care-pathway backbone that connects diagnostics, patient engagement, and clinical operations across eye care settings
Dry eye disease has moved from a symptom-led complaint to a chronic, multifactorial condition that clinicians increasingly manage as a longitudinal care pathway. That shift is pushing practices to rethink how they capture diagnostics, document treatment response, educate patients, and coordinate follow-ups across multiple touchpoints. As a result, dry eye software is no longer viewed as a simple add-on to imaging devices or an isolated screening module; it is becoming the connective layer that links clinical data, patient engagement, and operational throughput.At the same time, consumer expectations are reshaping the care experience. Patients want clearer explanations, visual evidence of improvement, and frictionless follow-up. Clinicians want tools that shorten the time between diagnostic insight and a documented plan, without multiplying clicks or introducing disconnected systems. Administrators want predictable workflows, standardized protocols, and compliant data handling. Consequently, solution providers are being asked to deliver an integrated experience that spans intake, testing, grading, treatment planning, and outcomes tracking.
This executive summary frames the most important forces shaping dry eye software today, highlighting where adoption is accelerating, where implementation risk still appears, and what decision-makers should prioritize to build durable clinical and commercial advantage. It sets the stage for understanding how technology choices made now can influence patient satisfaction, clinical consistency, and the ability to differentiate services in an increasingly competitive eye care environment.
Platform expectations, interoperability demands, and AI-assisted consistency are reshaping dry eye software from a reporting tool into an end-to-end workflow engine
The competitive landscape is being transformed by the convergence of diagnostic sophistication and workflow pragmatism. Historically, many solutions emphasized capturing measurements and producing reports. Now, the center of gravity is shifting toward orchestrating the entire visit and the months that follow. Platforms are being redesigned to reduce manual steps, auto-populate findings, and generate clinically credible narratives that can be reused for documentation and patient communication.A second shift is the rapid normalization of interoperability expectations. Decision-makers increasingly view stand-alone databases as liabilities because they create duplicate entry, complicate analytics, and limit scaling across locations. As EHR and practice-management ecosystems mature, dry eye tools are expected to exchange data reliably, align with identity management policies, and support role-based access across clinical and administrative teams. In parallel, vendor roadmaps are prioritizing APIs, structured exports, and integration with imaging devices and diagnostic instruments, not merely PDF outputs.
Artificial intelligence is also changing what “software” means in this category. Rather than treating AI as a headline feature, leading offerings are embedding it into segmentation of glands, staining pattern interpretation support, automated grading prompts, and visit-to-visit comparisons. The practical benefit is less about replacing clinicians and more about standardizing assessments across providers, reducing variability, and enabling faster decision-making for high-volume practices.
Finally, the care model itself is shifting. Dry eye clinics, optometry groups, and ophthalmology practices are increasingly offering bundled service lines that include in-office procedures, subscription-like replenishment of consumables, and ongoing education. Software is being asked to support that service design, including treatment protocols, patient reminders, consent capture, and outcomes tracking that can justify continued care. Taken together, these shifts are pushing the market from tool-based purchasing to platform-based decision-making, where usability, integration, and evidence generation are decisive differentiators.
United States tariff dynamics in 2025 may reshape dry eye software adoption via hardware-linked budgets, vendor packaging strategies, and deployment resilience needs
The 2025 tariff environment in the United States is poised to influence dry eye software purchasing decisions indirectly but meaningfully, largely through hardware-adjacent cost pressures and procurement caution. While software itself is typically delivered digitally, many deployments are tied to diagnostic devices, imaging systems, specialized cameras, and in-office treatment equipment that may rely on globally sourced components. When tariffs raise the landed cost of these systems or their replacement parts, clinics and health systems often delay upgrades, extend service contracts, or consolidate vendors, which can slow the pace of new software rollouts attached to new device installations.These pressures are likely to shift buying criteria toward solutions that are more device-agnostic, integration-friendly, and able to unify data from mixed fleets of instruments. Practices that postpone capital purchases still want to improve patient throughput and documentation quality, so they may prioritize software that can retrofit value onto existing devices through connectivity, standardized forms, and analytics rather than requiring a tightly coupled hardware refresh. In effect, tariff-driven cost sensitivity can increase the appeal of modular platforms and subscription models that spread spend over time.
Tariffs may also change vendor operating models. Providers of dry eye software that bundle hardware, implementation, and training could face margin compression if hardware costs rise faster than clinics’ willingness to pay. As a result, vendors may rebalance toward software-led value propositions, invest more heavily in remote onboarding and in-app guidance, and package tiers that make adoption viable for smaller practices without large upfront commitments.
From a risk perspective, tariff uncertainty can amplify supply chain variability for devices, which in turn can disrupt implementation schedules. That makes deployment planning, integration testing, and change management more important than ever. Buyers will benefit from contracting approaches that clarify delivery timelines, define data portability, and ensure continuity of support regardless of device availability. In this environment, resilience becomes a competitive advantage: solutions that can maintain consistent workflows despite hardware procurement volatility are better positioned to win trust and renewals.
Segmentation signals show adoption diverges by deployment preference, service intensity, organization scale, end-user workflow, and application depth across care pathways
Segmentation patterns reveal that dry eye software demand is not monolithic; it depends on how care is delivered, how value is captured, and what operational constraints exist. Across the segmentation landscape, offerings aligned to cloud deployment are gaining momentum because multi-location groups want centralized updates, easier role management, and simplified analytics. At the same time, on-premises implementations remain relevant for organizations with strict IT governance, bespoke integration requirements, or heightened sensitivity to data residency and uptime control, particularly when the software must operate seamlessly alongside local imaging networks.When viewed through the lens of component segmentation, the balance between software functionality and services is shifting. Buyers increasingly expect implementation, workflow design, data migration support, and ongoing optimization to be part of the overall solution, not an afterthought. This is especially true when teams want to standardize dry eye protocols across providers or align documentation with internal compliance practices. Consequently, vendors that can operationalize best practices-through templates, configurable pathways, and measurable adoption milestones-tend to reduce time-to-value and increase stickiness.
Organization size segmentation further clarifies purchasing priorities. Large enterprises and multi-site eye care groups often prioritize interoperability, governance, auditability, and the ability to benchmark performance across locations and clinicians. Smaller practices, by contrast, may place higher weight on ease of onboarding, intuitive patient education outputs, and a rapid path to incorporating dry eye services without adding administrative burden. This difference shapes feature adoption: enterprises lean into analytics and standardization, while smaller clinics often emphasize visit efficiency and revenue-cycle-friendly documentation.
End-user segmentation also influences product-market fit. Hospitals and integrated delivery networks often require formal security reviews, structured interfaces, and alignment with enterprise IT standards, which can elongate sales cycles but produce stable deployments. Specialty clinics and ophthalmology centers typically move faster, focusing on differentiating the patient experience and scaling procedural offerings. Optometry practices often value tools that elevate patient understanding during routine exams and convert appropriate patients into longitudinal care programs. Across these end-user contexts, the most successful solutions are those that adapt to the care setting rather than forcing the same workflow everywhere.
Finally, application segmentation underscores an important evolution: the category is moving beyond screening and documentation toward treatment planning, follow-up management, and outcomes tracking. As a result, solutions that provide longitudinal comparisons, integrate patient-reported symptoms, and support consistent grading frameworks are becoming more strategically important. Decision-makers are increasingly looking for platforms that can connect diagnostic findings to actionable next steps and then demonstrate whether those steps are working over time, reinforcing both clinical confidence and patient trust.
Regional adoption varies with care models and regulation, as the Americas emphasize scalable workflows while EMEA and Asia-Pacific prioritize compliance fit and adaptable deployment
Regional dynamics reflect differences in care delivery models, reimbursement environments, digital maturity, and patient expectations, all of which shape how dry eye software is evaluated and deployed. In the Americas, demand is closely tied to practice differentiation and operational throughput, with strong emphasis on demonstrating value through efficient visits and clear patient communication. Multi-location consolidation and the growth of specialized dry eye clinics increase the need for standardized protocols and shared reporting, making interoperability and governance key purchasing themes.In Europe, the Middle East, and Africa, adoption often hinges on navigating heterogeneous regulatory expectations and varied health system structures. Buyers frequently weigh data protection and clinical documentation rigor alongside usability, particularly when solutions must function across multiple countries or languages. Additionally, disparities in procurement processes between public and private providers can influence deployment approaches, creating opportunities for vendors that offer flexible implementation pathways and configurable workflows that can be tailored to local clinical norms.
In Asia-Pacific, expanding access to eye care, fast-growing private clinic networks, and rising consumer awareness of ocular surface health are fueling interest in streamlined digital workflows. Many organizations are leapfrogging toward cloud-first architectures, especially where multi-site expansion is rapid and centralized oversight is a priority. At the same time, the region’s diversity in infrastructure and purchasing patterns means successful solutions must perform reliably across a range of connectivity conditions and support fast training cycles for busy clinical teams.
Across all regions, patient expectations are converging toward more transparent, visual, and continuous care experiences, but the path to achieving that goal differs. Vendors that localize training, provide adaptable integrations, and support region-specific compliance needs are better positioned to scale. Meanwhile, providers that define a consistent care model-what is measured, how it is explained, and how follow-up is managed-can use software to deliver a repeatable experience regardless of geography.
Company competition centers on ecosystem integration, clinically credible outputs, and ownership of the visit-to-follow-up workflow through software-first and device-led strategies
Company strategies in dry eye software increasingly cluster around three differentiators: ecosystem connectivity, clinical credibility, and workflow ownership. Established ophthalmic technology suppliers often leverage device footprints to distribute software that ties directly into diagnostic instruments, creating a seamless capture-to-report experience. This approach can be compelling for clinics seeking tight integration and consistent outputs, though buyers are paying closer attention to data portability and the ability to incorporate third-party devices as instrument fleets diversify.Specialized software-first providers compete by delivering configurable protocols, patient engagement tools, and analytics that can sit above multiple devices and unify the patient record. These vendors frequently emphasize rapid iteration, UI improvements, and integration breadth, positioning their platforms as the operational hub for dry eye service lines rather than a single-instrument companion application. Their success increasingly depends on implementation excellence and the ability to demonstrate measurable workflow improvements within weeks, not months.
Another notable pattern is the growing emphasis on outcomes storytelling. Companies that enable before-and-after comparisons, longitudinal dashboards, and consistent grading frameworks help clinicians communicate progress and justify continued care. This capability matters because dry eye management often requires multiple visits and combinations of therapies; software that makes the journey visible can improve adherence and reduce confusion. As competition increases, the ability to translate clinical data into patient-friendly narratives becomes a commercial advantage.
Partnership activity is also reshaping competitive positioning. Integrations with EHR vendors, imaging platforms, and patient communication tools are becoming table stakes, while collaborations with diagnostic and therapeutic device manufacturers can accelerate adoption in procedure-heavy clinics. In parallel, security posture and compliance readiness are becoming more prominent differentiators as enterprise buyers apply stricter vendor risk reviews. In this environment, companies that pair credible clinical content with robust IT readiness and integration depth tend to earn larger deployments and longer relationships.
Leaders can win by standardizing care pathways, insisting on interoperability, adopting practical AI, aligning pricing to growth, and operationalizing outcomes discipline
Industry leaders can strengthen their position by treating dry eye software as a service-design decision rather than a purely technical purchase. Start by defining a standardized care pathway that specifies which diagnostics are captured, how severity is graded, what education is delivered, and when follow-ups occur. When the clinical playbook is clear, software selection becomes easier because the evaluation can focus on how well the platform operationalizes that pathway across providers and locations.Next, prioritize interoperability and data governance early in the buying process. Require clear answers on how data moves into and out of the platform, what structured exports are available, and how identity, roles, and audit logs are managed. This reduces the risk of future lock-in and enables cross-site benchmarking. It also supports smoother clinician adoption because integrations minimize duplicate entry and preserve workflow momentum.
Leaders should also evaluate AI features with a practical lens. The most valuable capabilities are those that reduce variability and save time without obscuring clinical judgment, such as guided grading prompts, automated comparisons, and quality checks that flag inconsistent documentation. Pair these capabilities with strong training and change management so that teams understand when to rely on automation and when to override it.
Commercially, consider packaging and pricing structures that align with clinic growth. Subscription models can support expansion and reduce tariff-related capital budgeting friction when software is associated with new hardware purchases. However, contracts should be specific about implementation support, uptime expectations, and data portability to protect operational continuity.
Finally, build an outcomes discipline. Establish a baseline of symptom reporting, objective measures, and patient education touchpoints, then use the software to track progress consistently. This not only improves care quality and patient trust but also helps leaders identify which protocols perform best across clinician teams. Over time, the ability to demonstrate consistent, repeatable outcomes becomes a differentiator that is difficult for competitors to replicate.
A triangulated methodology blending stakeholder interviews with rigorous secondary validation clarifies real-world adoption drivers, risks, and decision criteria
The research methodology integrates structured secondary research with primary engagement to capture both the technology trajectory and the operational realities of adoption. Secondary research focused on regulatory developments, software deployment practices in healthcare IT, cybersecurity and privacy considerations, and publicly available company materials such as product documentation, integration notes, and security statements. This foundation was used to map the competitive landscape and identify the most relevant decision factors shaping procurement.Primary research incorporated interviews and discussions with stakeholders across the ecosystem, including clinical leaders involved in dry eye service lines, practice administrators responsible for workflow and staffing, and executives and product leaders from solution providers. These conversations were used to validate how software is evaluated in real purchasing cycles, what implementation challenges recur, and which features most directly influence utilization after go-live.
Analytical work emphasized triangulation and consistency checks. Insights from interviews were cross-validated against observed product capabilities, documented integration approaches, and patterns in customer requirements. Where perspectives diverged, additional validation was sought to distinguish between aspirational roadmaps and currently deliverable functionality.
Finally, the methodology applied a segmentation lens to ensure findings reflect meaningful differences in deployment context and buyer expectations. This approach supports decision-makers by translating broad trends into practical implications, highlighting what tends to matter most depending on organizational structure, care setting, and operational priorities.
Dry eye software maturity is shifting value toward interoperable, resilient workflow platforms that enable consistent care delivery and longitudinal outcomes visibility
Dry eye software is becoming central to how ocular surface care is delivered, documented, and sustained over time. As the category matures, the most important value is shifting from generating attractive reports to orchestrating consistent workflows, integrating diverse diagnostic inputs, and enabling longitudinal outcomes visibility. This evolution favors solutions that are interoperable, implementation-ready, and designed around the realities of clinic operations.Looking ahead, external pressures such as tariff-driven hardware cost volatility and stricter enterprise security expectations will further reward platforms that are flexible and resilient. Buyers will increasingly seek software that can deliver value even when device upgrades are delayed, and vendors will be pushed to prove rapid time-to-value through strong onboarding, integration depth, and measurable workflow impact.
Ultimately, the organizations that succeed will be those that combine a clear clinical pathway with tools that make that pathway repeatable at scale. By aligning technology decisions with patient communication, provider consistency, and operational discipline, leaders can turn dry eye care into a differentiated, high-trust service line that performs reliably across settings.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Dry Eye Software Market
Companies Mentioned
The key companies profiled in this Dry Eye Software market report include:- Alcon Inc.
- AMO
- Canon Inc.
- Carl Zeiss Meditec AG
- Costruzione Strumenti Oftalmici S.p.A.
- CSO Italia S.r.l.
- Ellex Medical Lasers Ltd.
- Essilor International S.A.
- EyeScience, Inc.
- Haag-Streit AG
- iScience Interventional Ophthalmology, Inc.
- Johnson & Johnson Vision Care, Inc.
- Lumenis Ltd.
- Medmont International Pty Ltd
- Nidek Co., Ltd.
- Notal Vision Ltd.
- Ocuco Limited
- OCULUS Optikgeräte GmbH
- Optovue, Inc.
- Righton Eye Co., Ltd.
- RVL Medical
- SBM Sistemi S.p.A.
- TearLab Corporation
- TearScience, Inc.
- Tomey Corporation
- Topcon Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
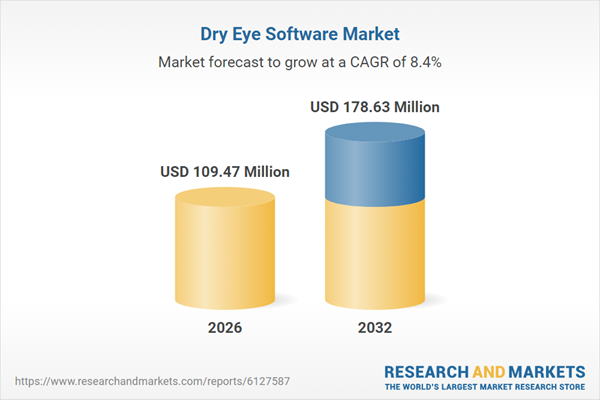
| Estimated Market Value ( USD | $ 109.47 Million |
| Forecasted Market Value ( USD | $ 178.63 Million |
| Compound Annual Growth Rate | 8.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |