Speak directly to the analyst to clarify any post sales queries you may have.
Medical grade nitinol materials redefine minimally invasive performance, elevating metallurgy, process control, and compliance into strategic priorities
Medical grade nitinol materials sit at the intersection of metallurgy, precision processing, and clinical performance. As a nickel-titanium alloy family engineered for superelasticity and shape memory, nitinol enables minimally invasive devices to deliver repeatable force, kink resistance, and recoverable strain in anatomies where stainless steel or cobalt-chrome may be too stiff or fatigue-limited. Yet the value of nitinol in healthcare is not confined to the alloy chemistry alone; it is realized through tightly controlled melting, thermomechanical processing, surface finishing, and verification protocols that collectively determine transformation behavior, corrosion performance, and fatigue life.In parallel, the industry has moved from viewing nitinol as a specialty material to treating it as a strategic platform technology across cardiovascular, neurovascular, peripheral vascular, and structural heart indications. This shift has elevated expectations for supply continuity, traceability, and standardization, while also increasing scrutiny around nickel release, particulate generation, and the consistency of surface states after electropolishing, passivation, or proprietary treatments.
Against this backdrop, the executive summary frames the market environment through the lens of technology maturity, regulatory and quality requirements, and the evolving procurement calculus that device manufacturers face. The discussion emphasizes how process capability and documentation increasingly differentiate suppliers, and why the most consequential decisions now center on qualification speed, change control discipline, and end-to-end risk management rather than raw material access alone.
Device complexity, regulatory rigor, and advanced manufacturing are transforming nitinol into a tightly managed, performance-engineered supply chain
The landscape for medical grade nitinol has undergone transformative shifts driven by converging forces in device design, manufacturing technology, and regulatory expectations. First, device architectures increasingly rely on complex, multi-stage deformation profiles rather than simple expansion or straightening, which in turn demands tighter control over transformation temperatures, hysteresis, and plateau stresses. As a result, manufacturers are engineering not only the device geometry but also the stress-strain response, placing new importance on heat-treatment recipes, cold work levels, and lot-to-lot reproducibility.Second, the industry has pivoted toward deeper vertical integration and more collaborative development models. Where nitinol procurement once centered on purchasing wire or tubing to a specification, leading programs now require supplier participation in design for manufacturability, fatigue testing strategy, and surface integrity optimization. This has created a more interdependent ecosystem in which upstream melt quality and downstream finishing steps are evaluated as a single performance chain, particularly for thin-walled tubes and fine wires where microstructural variability can rapidly translate into durability risk.
Third, advanced manufacturing and inspection capabilities are reshaping competitive standards. Laser cutting, femtosecond processing, and increasingly sophisticated debris control expectations have raised the bar for surface quality and edge integrity. At the same time, non-destructive evaluation, tighter dimensional metrology, and expanded documentation expectations are becoming routine requirements in qualification packages. Consequently, differentiation is shifting toward process windows that are demonstrably stable, accompanied by robust statistical control and transparent change management.
Finally, sustainability and resilience considerations have entered the conversation. While patient safety and performance remain paramount, procurement teams are more actively assessing multi-sourcing strategies, regional redundancy, and the carbon and waste footprint of finishing operations. Taken together, these shifts are accelerating professionalization across the supply chain and rewarding organizations that can combine materials science expertise with operational excellence and regulatory readiness.
Tariffs in 2025 reshape landed-cost logic and qualification risk, pushing nitinol programs toward resilient, documentation-first supply strategies
United States tariffs in 2025 introduce a cumulative set of pressures that reverberate across medical grade nitinol sourcing, conversion, and downstream device manufacturing. Even when certain medical products or materials are exempted or treated differently across tariff schedules, the practical impact often emerges through adjacent categories, intermediate goods, and the broader cost of industrial inputs. For nitinol, where value is added through multiple conversion steps-melting, forging or hot working, drawing, tube making, laser processing, and surface finishing-tariff exposure can compound as materials cross borders during different stages of qualification and scale-up.In response, procurement strategies are shifting from price negotiation toward total landed-cost engineering and risk-adjusted sourcing. Companies are revisiting incoterms, buffer inventory policies, and dual-sourcing plans not merely to reduce unit cost but to protect development timelines from customs delays and administrative variability. Moreover, tariff-driven cost pressure can influence decisions about whether to qualify alternate product forms or dimensions that are more readily available domestically, even if they require design adjustments or additional verification.
The tariff environment also amplifies the importance of traceability and documentation discipline. When supply chains are re-routed or when intermediate processing is moved to different regions, maintaining consistent certification packets, heat/lot genealogy, and validated equivalence of finishing steps becomes critical to regulatory compliance. Any perceived “material change” may trigger revalidation expectations, particularly for implants and fatigue-critical components.
Over time, the cumulative impact is likely to accelerate localization of selected processing steps, encourage longer-term supply agreements, and increase interest in domestic capacity expansion for high-value conversion operations. However, because nitinol manufacturing excellence depends on specialized equipment, know-how, and proven quality systems, the transition is not instantaneous. Industry leaders will be those who proactively model tariff scenarios, qualify resilient supply lanes, and protect clinical and commercial schedules through disciplined change control.
Segmentation reveals that form factor, device application, care setting, and finishing route jointly dictate nitinol specifications and supplier selection
Key segmentation insights begin with recognition that medical grade nitinol is not a monolithic input; it is a family of material forms and processing conditions tuned to device-specific performance requirements. Across product types such as wire, tubing, strip, sheet, and bar or rod, the value drivers differ materially. Wire-centric applications often prioritize ultra-consistent diameter control, surface cleanliness, and fatigue behavior under bending and torsion, while tubing applications place emphasis on concentricity, wall uniformity, laser-cut response, and post-processing surface integrity. Sheet and strip, in contrast, are more closely tied to stamping or forming behavior and may require specialized heat-treatment pathways to preserve functional properties after deformation.When viewed through application segmentation spanning stents, guidewires, catheters, heart valve frames, embolic protection, orthopedic implants, and dental devices, the most important insight is how design intent dictates the acceptable variation envelope. Stents and valve frames generally demand a tightly engineered superelastic response and exceptional durability under cyclic loading, which elevates the importance of transformation temperature control and surface finish consistency. Guidewires and catheter reinforcement components may place stronger weight on torque response, kink resistance, and interface compatibility with polymer jackets, making surface treatments and adhesion behavior more consequential.
End-use segmentation across hospitals, ambulatory surgical centers, and specialty clinics indirectly shapes material requirements by influencing device design priorities. In high-throughput settings where procedural consistency matters, device manufacturers tend to favor performance predictability and ease of deployment, driving tighter upstream specifications and a preference for suppliers with mature validation evidence. In specialty settings that adopt novel procedures earlier, manufacturers may push more experimental geometries and mixed-material assemblies, increasing the need for iterative prototyping capability and responsive engineering support.
Finally, segmentation by processing route and finishing approach-such as vacuum melting practices, cold work levels, shape-setting heat treatments, electropolishing, passivation, and coated surfaces-highlights that “medical grade” is ultimately established by controlled outcomes rather than a single label. The strongest opportunities typically sit where suppliers can couple repeatable functional performance with scalable finishing quality and robust documentation, reducing qualification friction for device manufacturers across multiple product families.
Regional patterns show how manufacturing maturity, compliance regimes, and supply resilience shape nitinol qualification across global medtech hubs
Regional dynamics for medical grade nitinol reflect differences in device manufacturing concentration, regulatory pathways, and the maturity of specialty metals ecosystems. In the Americas, a strong base of cardiovascular and neurovascular device innovation sustains demand for tight specification control, rapid prototyping support, and audit-ready quality systems. Manufacturers in this region tend to emphasize supply continuity and change control, particularly for fatigue-critical implants and delivery systems where requalification timelines can be commercially disruptive.Across Europe, the market environment is shaped by a mix of established medtech hubs and cross-border supply networks. Regional emphasis often falls on harmonized quality documentation, environmental and chemical compliance expectations, and a growing preference for suppliers that can demonstrate consistent surface integrity and cleanliness outcomes. In addition, the interaction between notified body expectations and internal risk management has encouraged more rigorous verification of material equivalence whenever processing locations or finishing recipes change.
The Middle East & Africa presents a different profile, where adoption is closely linked to healthcare infrastructure expansion, imported device availability, and the build-out of specialized clinical capabilities. While much of the nitinol value chain is supplied through global manufacturers, there is a rising focus on ensuring stable access to advanced devices and on building clinical programs that can support minimally invasive procedures, which indirectly sustains demand for reliable, globally qualified material inputs.
Asia-Pacific is characterized by rapid scaling of device manufacturing capacity and an expanding footprint in both component conversion and finished device production. As regional quality systems mature and export ambitions increase, there is heightened attention to traceability, international standards alignment, and process validation depth. At the same time, the region’s manufacturing agility supports faster iteration cycles, which can accelerate adoption of new nitinol forms and processing approaches when paired with strong compliance practices.
Taken together across the Americas, Europe, Middle East & Africa, and Asia-Pacific, regional insights point to a common trajectory: buyers are increasingly benchmarking suppliers on validation evidence, responsiveness during qualification, and the ability to support multi-regional compliance without introducing uncontrolled variation.
Company differentiation now hinges on validated consistency, lifecycle support, and change-controlled documentation that de-risks device qualification
Competitive positioning among key companies in medical grade nitinol is increasingly defined by controllable outcomes rather than broad capability claims. Leaders distinguish themselves through consistent transformation temperature control, stable mechanical response after shape setting, and proven fatigue performance supported by rigorous test methodologies. Just as importantly, they provide robust certification packets, transparent heat/lot genealogy, and disciplined change notification practices that align with medical device quality expectations.Another differentiator is the ability to support the full lifecycle from prototyping to scale. Companies that can deliver small-lot materials quickly-without compromising documentation quality-help device teams iterate faster during design verification. As programs move toward commercialization, the same suppliers must demonstrate process scalability, statistically controlled production, and repeatable finishing performance, especially for electropolished or otherwise surface-engineered components.
Value-added services also shape company insights. Integrated offerings that combine tube making, laser cutting readiness, debris control know-how, and finishing partnerships reduce handoffs that can introduce variability. In contrast, organizations that remain narrow in scope can still succeed when they are exceptional in a specific form factor, such as ultra-fine wire or thin-wall tubing, but they face increased scrutiny around how their material integrates into downstream processing.
Finally, strategic alignment with regulatory and clinical risk expectations is a key competitive lever. Companies that invest in application engineering support, standardized test protocols, and proactive failure analysis capabilities tend to become preferred partners for implantable and fatigue-critical devices. As a result, competitive strength is increasingly measured by qualification velocity, audit performance, and the capacity to manage change without disrupting device approvals or clinical outcomes.
Leaders can reduce qualification friction and supply risk by engineering functional specs, resilient sourcing plans, and cross-functional governance
Industry leaders can act decisively by treating medical grade nitinol as a managed performance system rather than a commodity. First, strengthen specifications around functional outcomes-such as transformation behavior, plateau stress, and fatigue performance-while explicitly linking those outcomes to controlled process parameters and inspection methods. This approach reduces ambiguity during supplier transitions and enables more efficient root-cause analysis when performance drifts.Next, build tariff- and disruption-aware sourcing strategies that prioritize qualification resilience. Dual sourcing is most effective when it is planned early, with harmonized test methods, matched finishing routes where feasible, and pre-agreed change control triggers. In parallel, establish clearer internal criteria for when a supply chain modification constitutes a material change requiring verification, validation, or regulatory notification.
Leaders should also invest in deeper collaboration across R&D, quality, and procurement. Cross-functional supplier scorecards that include documentation timeliness, process capability indices, nonconformance response speed, and audit readiness can prevent late-stage surprises. Where devices are fatigue-critical, align on standardized fatigue test conditions and acceptance frameworks to avoid inconsistent comparisons between development lots and production lots.
Finally, accelerate learning loops through structured prototyping and feedback. Capturing correlations between processing history, surface state, and in-device performance can guide more robust design margins and reduce sensitivity to minor lot-to-lot variation. Over time, organizations that institutionalize this knowledge-through design controls, supplier development programs, and validated equivalence strategies-will be better positioned to innovate while maintaining compliance and supply continuity.
A triangulated methodology links alloy processing to device performance, combining expert interviews with standards and technology intelligence
The research methodology integrates primary and secondary techniques to develop a decision-oriented view of medical grade nitinol materials, emphasizing technology, qualification practices, and supply chain dynamics. The work begins by framing the value chain from melting and conversion through finishing and device integration, allowing the analysis to connect upstream process controls with downstream performance and compliance outcomes.Primary research focuses on structured interviews with stakeholders across the ecosystem, including material suppliers, converters, contract manufacturers, quality and regulatory professionals, and device engineers. These discussions are used to validate practical qualification requirements, common failure modes, documentation expectations, and evolving procurement strategies under changing trade and compliance conditions.
Secondary research consolidates technical standards, regulatory guidance, patent and technology signals, corporate disclosures, and publicly available information related to process capabilities and material applications. The synthesis prioritizes consistency checks across sources and highlights areas where terminology differences can mask meaningful technical distinctions, such as “superelastic” specifications that vary by test method.
Throughout the study, triangulation is used to reduce bias by comparing perspectives across roles and regions, and by reconciling reported practices with documented standards and real-world manufacturing constraints. Findings are then organized into actionable themes-focused on segmentation, regional dynamics, and company positioning-so that readers can translate the analysis into qualification plans, supplier strategies, and product development decisions.
Nitinol’s future belongs to organizations that master process consistency, compliance discipline, and resilient supply chains for critical devices
Medical grade nitinol continues to gain strategic importance as minimally invasive therapies expand and device designs demand more engineered mechanical behavior. The most durable advantage no longer comes from merely accessing the alloy, but from controlling the full chain of value creation-composition discipline, thermomechanical processing, surface integrity, and documentation that supports regulatory confidence.As the landscape evolves, transformative shifts in manufacturing technology, compliance expectations, and supply chain resilience are redefining what “best in class” looks like. Tariff pressures and broader trade uncertainty further elevate the importance of total landed-cost planning, qualification-ready documentation, and multi-sourcing strategies that do not compromise performance consistency.
Ultimately, the winners in this environment will be organizations that treat nitinol as a performance platform supported by rigorous change control and cross-functional decision-making. By aligning engineering intent with procurement strategy and validated process capability, industry participants can reduce risk, accelerate qualification, and sustain innovation in high-stakes clinical applications.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Medical Grade Nitinol Materials Market
Companies Mentioned
The key companies profiled in this Medical Grade Nitinol Materials market report include:- Advanced Material Technologies GmbH
- Allegheny Technologies Incorporated
- Arthrex, Inc.
- Endosmart GmbH
- Furukawa Electric Co., Ltd.
- Johnson Matthey Plc
- Memry Corporation
- Neo-Metrics, Inc.
- Nitinol Devices & Components, Inc.
- SAES Getters S.p.A.
- SMST GmbH
- Special Metals Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
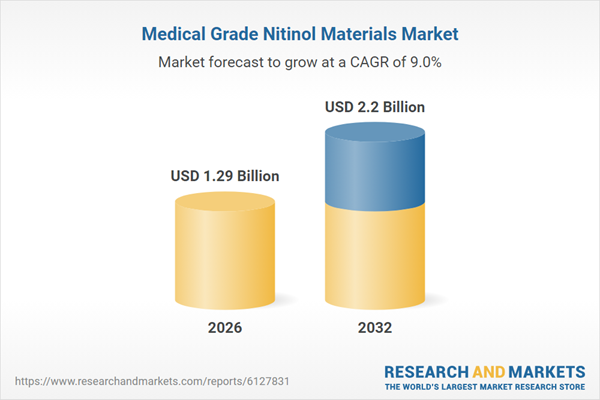
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 1.29 Billion |
| Forecasted Market Value ( USD | $ 2.2 Billion |
| Compound Annual Growth Rate | 9.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |