Speak directly to the analyst to clarify any post sales queries you may have.
Precision medical coatings are evolving into performance-critical interfaces that define clinical outcomes, device differentiation, and regulatory readiness
Precision medical coatings sit at the intersection of materials science, regulatory discipline, and patient outcomes. They are not simply surface finishes; they are engineered interfaces that control friction, biocompatibility, antimicrobial response, wear behavior, and chemical resistance under demanding use conditions. As device designers push toward smaller profiles, more complex geometries, and longer indwelling times, coating performance has become inseparable from product differentiation and clinical adoption.Across hospitals and outpatient settings, clinicians increasingly expect devices to glide smoothly, resist biofouling, and maintain predictable performance throughout a procedure or therapy cycle. This expectation is shaping procurement conversations as well as design requirements, particularly in catheters, guidewires, orthopedic implants, surgical instruments, and wound-care materials. In parallel, manufacturers face tighter tolerance windows and elevated scrutiny of leachables, extractables, and particulate generation, which places additional emphasis on coating selection, process control, and validation.
Against this backdrop, the market environment is being reshaped by supply chain constraints, shifting trade policies, and accelerated innovation in polymer systems, deposition methods, and surface functionalization. The executive summary that follows frames the most consequential forces influencing precision medical coatings today, highlighting how segmentation dynamics, regional realities, and competitive strategies are redefining what “best-in-class” looks like for both established leaders and emerging specialists.
From single-function finishes to multi-functional, regulated, and sustainable surfaces, precision coatings are being reinvented by technology and care delivery shifts
The precision medical coating landscape is undergoing a set of transformative shifts driven by technology convergence, changing clinical care models, and heightened quality expectations. One of the most significant changes is the move from single-function coatings toward multi-functional surfaces that combine lubricity with antimicrobial or anti-thrombogenic properties, or that pair wear resistance with controlled drug release. This shift is most visible where device dwell time increases and infection or clot risk must be actively managed, making surface engineering part of the therapy rather than an accessory to it.At the same time, coating development is increasingly guided by sustainability and risk-reduction imperatives. Concerns around persistent chemicals and tighter environmental stewardship expectations are prompting renewed attention to fluorinated chemistries, solvent systems, and waste streams. Even when specific chemistries remain viable, product stewardship programs, customer audits, and investor expectations are pushing manufacturers to document material choices, process emissions, and end-of-life considerations more rigorously.
Manufacturing strategy is also changing. As OEMs seek higher reliability and faster iteration, the coating process is being treated as a core manufacturing competence rather than a “black box” subcontracted step. This is catalyzing deeper partnerships between coating specialists and device companies, including co-development agreements, shared validation plans, and earlier involvement of coating engineers in design-for-manufacturability. In addition, advanced automation, in-line inspection, and data-driven process control are becoming differentiators, particularly for thin, uniform coatings on complex shapes where variability can undermine performance or regulatory submissions.
Finally, regulatory and clinical evidence expectations are reshaping how coatings are positioned. Stakeholders want clearer linkage between coating performance claims and measurable clinical benefits, supported by test methods that are reproducible and relevant to use conditions. As a result, the competitive arena is shifting from “who has the chemistry” to “who can industrialize, qualify, and defend the performance” across multiple manufacturing sites and changing supply chain conditions.
Tariff shifts in 2025 are poised to reshape coating inputs, qualification strategies, and total landed cost priorities across U.S.-linked supply chains
United States tariff actions and trade policy adjustments expected in 2025 are set to influence precision medical coating supply chains in practical, near-term ways. Even when finished medical devices receive preferential treatment or exemptions, upstream dependencies-such as specialty chemicals, monomers, additives, metal targets, deposition equipment, and precision components used in coating lines-can still be exposed to duty changes. The result is an indirect cost and availability impact that can be felt across qualification timelines and production continuity.A primary cumulative effect is the acceleration of dual-sourcing and regionalization strategies. Coating providers and OEMs are likely to intensify efforts to qualify alternate raw material suppliers, validate second-site manufacturing, and redesign bills of materials to reduce exposure to tariff-sensitive inputs. However, qualification is not a simple commercial exercise in medical markets; any change in chemistry, supplier, or process window can require additional biocompatibility work, stability testing, and documentation updates. Therefore, the tariff-driven push for supplier flexibility tends to increase the value of platforms that can accommodate multiple feedstocks with minimal performance drift.
Another impact is greater emphasis on total landed cost modeling rather than unit pricing. Tariffs interact with freight volatility, longer lead times, and inventory buffering, and they can amplify the cost of nonconformance when suppliers rush substitutions. In response, device manufacturers are expected to tighten supplier scorecards, seeking stronger evidence of change control discipline, raw material traceability, and contingency planning. This favors coating organizations that have mature quality systems, robust supplier qualification protocols, and an ability to provide documentation packages aligned with medical device regulatory expectations.
In parallel, tariffs can influence innovation pathways. When certain imported inputs become less predictable, R&D teams may prioritize formulations and deposition approaches that rely on more readily available domestic or tariff-resilient materials. Over time, this can shift the competitive balance toward coating technologies that are easier to localize, scale, and audit. The cumulative outcome is a market that rewards operational resilience and compliance-ready agility as much as it rewards breakthrough surface performance.
Segmentation signals show that coating type, material systems, deposition routes, and application demands are converging toward validation-ready performance platforms
Segmentation dynamics in precision medical coatings reveal how performance requirements, regulatory burden, and manufacturing practicality vary by coating type, material family, deposition approach, application area, and end-user expectations. In coating type terms, hydrophilic systems continue to gain attention where ultra-low friction and improved navigability are essential, while hydrophobic layers remain central to fluid repellency and contamination control in selected device environments. Antimicrobial surfaces are increasingly evaluated not only for efficacy but also for durability, safety profile, and compatibility with sterilization methods, and anti-thrombogenic strategies are being scrutinized for their stability and risk-benefit justification across different blood-contacting indications.Material choices show a similar pattern of specialization. Polymer-based coatings are frequently preferred where flexibility, tunable chemistry, and scalable application are required, while metallic and ceramic coatings are often selected for wear resistance, hardness, and barrier properties on implants and instruments. In high-performance use cases, hybrid or multilayer systems are being adopted to balance lubricity with mechanical robustness, acknowledging that a single layer rarely optimizes all needed attributes under real-world loading, cleaning, and sterilization cycles.
Process and deposition segmentation highlights the strategic trade-off between throughput and precision. Dip and spray methods remain valuable for many polymer coatings due to their manufacturability and cost control, yet they can demand advanced controls to manage thickness uniformity on complex geometries. Parylene deposition is often pursued for conformal barrier protection and electrical insulation, especially when pinhole-free coverage is essential. Plasma-based and vapor-phase approaches are gaining traction where surface activation, adhesion promotion, or ultra-thin functional layers are needed without adding bulk. In parallel, the industry is increasingly differentiating suppliers by their ability to validate processes, monitor critical parameters, and provide reproducible outputs across shifts, lines, and sites.
Application-based segmentation further clarifies demand drivers. Catheters and guidewires place a premium on lubricity and consistency, particularly as minimally invasive procedures expand. Implant-related applications elevate the importance of wear behavior, corrosion resistance, and long-term biocompatibility, while surgical tools and instruments often require coatings that withstand repeated reprocessing without degradation or particulate shedding. Medical electronics and sensors emphasize barrier and dielectric properties, reflecting the growth in connected care and monitoring. Meanwhile, wound care and healthcare textiles are drawing increased attention to antimicrobial durability, skin compatibility, and comfort.
End-user segmentation underscores how purchasing and qualification behavior differs. OEMs tend to prioritize platform scalability, documentation depth, and supply assurance, whereas specialized device innovators may seek rapid prototyping support, design collaboration, and flexible minimum order quantities. Contract manufacturers and coating service providers sit between these needs, often acting as the operational bridge that translates device design intent into stable, auditable coating processes. Across all segments, the common thread is the rising importance of validation readiness and change control as core elements of product value-not merely compliance obligations.
Regional realities across the Americas, Europe Middle East & Africa, and Asia-Pacific are redefining coating strategies through compliance, scale, and resilience needs
Regional dynamics in precision medical coatings reflect differences in medtech manufacturing density, regulatory pathways, labor and energy costs, and access to specialty materials. In the Americas, demand is strongly shaped by mature device manufacturing ecosystems and an emphasis on resilient supply chains. Manufacturers in this region often prioritize process transparency, robust documentation, and rapid scale-up support, especially for minimally invasive device categories. Nearshoring and second-site qualification strategies are reinforcing the role of coating partners that can deliver consistent outputs while meeting stringent quality and traceability expectations.Across Europe, the Middle East, and Africa, regulatory rigor and sustainability pressures play an outsized role in coating decisions. European manufacturers and healthcare stakeholders tend to scrutinize chemical stewardship and environmental controls, which influences solvent selection, waste handling, and documentation of material compliance. In addition, the region’s strong implant and orthopedic footprint sustains demand for wear-resistant and corrosion-resistant surfaces, while ongoing modernization of healthcare infrastructure in parts of the Middle East and Africa supports growth in device adoption, frequently coupled with procurement frameworks that emphasize reliability and lifecycle value.
In Asia-Pacific, scaling capability and manufacturing breadth shape the competitive environment. The region combines advanced materials expertise with expanding device production capacity, and it often serves as both an innovation engine and a high-volume manufacturing base. As local regulatory systems mature and export-oriented manufacturers align with global quality standards, coating suppliers that can deliver repeatability, high throughput, and strong technical support are positioned to benefit. At the same time, cross-border supply dependencies for specialty inputs remain a strategic consideration, making flexibility in raw materials and multi-site production planning increasingly important.
Taken together, these regional patterns reinforce a central theme: successful coating strategies increasingly depend on matching performance requirements to region-specific realities in compliance, sourcing, and operational execution. Companies that build regional redundancy without fragmenting their quality systems are better equipped to sustain growth and meet customer expectations.
Competitive advantage now hinges on validated coating platforms, multi-site manufacturability, and lifecycle risk management rather than chemistry alone
Competitive differentiation among key companies in precision medical coatings is increasingly defined by the ability to pair materials expertise with industrial discipline. Leaders distinguish themselves through broad technology portfolios that span lubricious, barrier, antimicrobial, and wear-resistant solutions, supported by validated processes that can be transferred across manufacturing lines with predictable outcomes. Just as important is the capability to support customers from early-stage design iterations through commercialization, including test method development, fixture design, and support for documentation packages used in regulatory submissions.Another defining trait is how companies manage risk across the coating lifecycle. Strong competitors demonstrate mature change control programs, supplier qualification rigor, and proactive monitoring for raw material variability. This is particularly relevant as device companies seek to avoid revalidation delays when inputs change. Firms that can offer platform approaches-where a family of coatings shares a consistent process architecture-often reduce qualification friction for customers while still enabling tailoring for specific device geometries and use conditions.
Manufacturing excellence is also becoming a headline differentiator. Companies investing in automation, in-line inspection, and data capture improve reproducibility and reduce scrap, which matters for high-volume categories and for precision-coated components with tight tolerances. In parallel, organizations with global footprints or multi-regional partnerships can offer better continuity planning, which is increasingly valued amid logistical volatility and evolving trade policy.
Finally, service model maturity separates commodity providers from strategic partners. The strongest companies combine technical consultative selling with disciplined program management, clear validation roadmaps, and realistic scale-up plans. As OEMs demand faster development cycles without compromising patient safety, coating suppliers that align R&D, quality, and operations under a unified customer delivery model are best positioned to win long-term programs.
Leaders can win by standardizing coating platforms, hardening dual-sourcing qualification, and elevating durability-focused validation and automation
Industry leaders can strengthen their position by treating coatings as a strategic system that links clinical performance, manufacturing stability, and regulatory readiness. One priority is to standardize around a manageable set of coating platforms while preserving room for application-specific tuning. Platform standardization reduces qualification burden, speeds up tech transfer, and improves procurement leverage, yet it must be paired with clear boundaries for allowable raw material substitutions and process window adjustments.To improve resilience in the face of tariff and logistics uncertainty, leaders should build qualification pathways for dual sourcing early, before disruptions force rushed changes. This includes pre-approved alternates for critical inputs, documented comparability protocols, and periodic supplier stress tests. In parallel, organizations should strengthen total landed cost governance by connecting sourcing, quality, and engineering data into a single decision framework that accounts for lead time risk, documentation readiness, and nonconformance exposure.
Innovation programs should increasingly focus on durability and real-world performance retention rather than initial benchmark results alone. For lubricious and antimicrobial systems in particular, durability under sterilization, storage, and simulated use should be emphasized, since performance decay can undermine both clinical outcomes and brand trust. Leaders can also accelerate adoption by aligning test methods with clinically relevant failure modes, improving the credibility of performance claims and reducing back-and-forth during customer audits.
Operationally, investments in automation and in-line metrology should be paired with quality-by-design thinking. By identifying critical process parameters and building control strategies around them, coating operations can reduce variability and create more robust validation packages. Finally, leaders should deepen cross-functional collaboration among R&D, regulatory, sourcing, and manufacturing teams so that material choices, documentation plans, and scale-up assumptions remain aligned throughout development and commercialization.
A rigorous methodology combining expert interviews, technical validation, and triangulated secondary review builds decision-ready coating intelligence
The research methodology for this report combines structured primary engagement with rigorous secondary analysis to develop a coherent view of precision medical coating technologies, adoption patterns, and competitive positioning. Primary inputs include interviews and discussions with stakeholders across the value chain, such as medical device manufacturers, coating service providers, materials suppliers, and subject matter experts in surface engineering, quality systems, and regulatory compliance. These engagements are designed to capture practical decision criteria, qualification bottlenecks, and emerging performance requirements across major application areas.Secondary research incorporates a broad review of publicly available and professionally accessible materials, including company disclosures, product and technology documentation, regulatory and standards guidance, patent activity signals, and technical literature relevant to coating chemistries and deposition processes. This step is used to validate terminology, map technology evolution, and triangulate claims about performance attributes and manufacturability constraints.
Analytical framing emphasizes consistency and traceability. Insights are cross-checked across multiple inputs to reduce single-source bias, and themes are validated against observable industry behaviors such as investment patterns, partnership announcements, and manufacturing footprint changes. Segmentation analysis is structured to reflect how buyers actually evaluate coatings, linking coating type and deposition choices to application-specific needs, validation requirements, and supply chain realities.
Finally, the report’s conclusions are reviewed for internal coherence, ensuring that regional observations, tariff implications, and competitive strategies align with the underlying technology and regulatory context. This approach supports decision-ready insights without relying on speculative assumptions.
Coatings are becoming strategic manufacturing and clinical assets, rewarding companies that combine surface science, validation discipline, and resilient supply execution
Precision medical coatings have moved from supportive materials to strategic enablers of device performance, safety, and manufacturability. The market’s direction is being set by multi-functional surface demand, tighter expectations around documentation and change control, and an industry-wide push for supply chain resilience. These forces are raising the bar for what customers expect from coating partners: not just a formulation, but a validated, scalable, auditable process that performs consistently over time.As trade policy uncertainty and upstream input volatility persist, the ability to qualify alternates efficiently and sustain performance across multiple sites becomes a defining capability. Meanwhile, technology shifts toward conformal barriers, advanced surface activation, and durable lubricity are expanding the design space for next-generation devices, but also increasing the need for disciplined validation planning.
Organizations that align innovation with manufacturability, pair platform strategies with targeted customization, and invest in quality-by-design controls will be best positioned to compete. In this environment, success belongs to those who can translate surface science into reliable production outcomes while meeting the growing expectations of regulators, clinicians, and procurement teams alike.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Precision Medical Coating Market
Companies Mentioned
The key companies profiled in this Precision Medical Coating market report include:- 3M Company
- Abbott Laboratories
- AST Products, Inc.
- Axalta Coating Systems, LLC
- BASF SE
- Biocoat, Inc.
- Covalon Technologies Ltd.
- DSM-Firmenich AG
- Evonik Industries AG
- Formacoat LLC
- Freudenberg Medical, LLC
- GE HealthCare Technologies Inc.
- Harland Medical Systems, Inc.
- Henkel AG & Co. KGaA
- Heraeus Holding GmbH
- Hydromer, Inc.
- Ionbond LLC
- Medicoat AG
- Merit Medical Systems, Inc.
- Momentive Performance Materials Inc.
- PPG Industries, Inc.
- Precision Coating Company, Inc.
- Surmodics, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
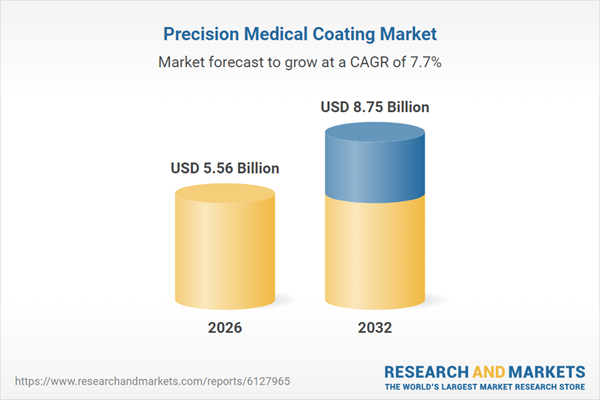
| Estimated Market Value ( USD | $ 5.56 Billion |
| Forecasted Market Value ( USD | $ 8.75 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |