Speak directly to the analyst to clarify any post sales queries you may have.
Antitumor ADC drugs are redefining precision oncology by pairing targeted antibodies with potent payloads, elevating expectations for efficacy, safety, and scalable delivery
Antitumor antibody-drug conjugates (ADCs) have moved from a compelling scientific concept to a cornerstone modality in oncology development, reshaping how stakeholders think about targeted cytotoxic delivery. By uniting the selectivity of monoclonal antibodies with the potency of small-molecule payloads through engineered linkers, ADCs aim to widen the therapeutic window in tumor settings where conventional chemotherapy and even some targeted agents face constraints. As clinical experience expands, the field is evolving beyond first-generation constructs toward designs that optimize stability in circulation, controlled payload release at the tumor site, and improved activity in heterogeneous tumors.This momentum is being reinforced by a broader shift in oncology practice toward precision-driven regimens, combination strategies, and biomarker-informed patient selection. ADCs are increasingly evaluated not only as late-line options but also for earlier lines of therapy, where expectations for safety, durability, and quality-of-life outcomes are higher. At the same time, pipeline breadth is expanding across both solid tumors and hematologic malignancies, supported by advances in target biology, antibody engineering, conjugation chemistry, and manufacturing analytics.
Against this backdrop, executives face a different kind of complexity than in earlier biopharma cycles. Success depends on orchestrating discovery, clinical differentiation, scalable CMC readiness, global supply continuity, and payer-aligned value narratives-often simultaneously. This executive summary synthesizes the forces shaping the antitumor ADC drugs landscape, highlights how segmentation and regional dynamics influence strategy, and frames the practical implications of policy and trade conditions that will matter in 2025 and beyond.
Platform-driven R&D, smarter linker-payload engineering, and manufacturing as a competitive weapon are reshaping how antitumor ADC leaders win
The antitumor ADC landscape is undergoing transformative shifts driven by both scientific breakthroughs and changing competitive behavior. One of the most consequential changes is the pivot from single-asset programs to portfolio thinking, where companies build platforms around conjugation technologies, linker chemistries, and payload classes that can be rapidly redeployed across targets. This platform orientation shortens iteration cycles, improves learnings transfer between programs, and strengthens partnering leverage.In parallel, the modality is maturing from “targeted chemotherapy” toward a more nuanced therapeutic category with design choices that directly shape clinical performance. Developers are using refined linker technologies to balance plasma stability with efficient intracellular release, while also optimizing drug-antibody ratio consistency and controlling aggregation risk. Payload innovation is similarly dynamic, with continued interest in microtubule inhibitors alongside DNA-damaging agents and emerging immune-stimulatory payload concepts that may enable broader antitumor activity when combined with checkpoint inhibition.
Competition is also shifting geographically and structurally. A growing number of cross-border licensing deals and co-development partnerships have accelerated, reflecting how ADC programs benefit from complementary strengths-discovery in one region, clinical execution in another, and manufacturing scale-up supported by specialized CDMOs. At the same time, regulators and payers are raising the bar for evidence in crowded indications, pushing developers to design smarter trials, incorporate patient-relevant endpoints, and establish differentiation beyond response rates alone.
Finally, manufacturing has become a strategic battleground rather than a back-end function. As ADC volumes increase and product complexity remains high, sponsors are investing earlier in process robustness, analytical characterization, containment capabilities for highly potent payloads, and dual sourcing strategies. These shifts collectively indicate a market where differentiation hinges on end-to-end execution and where science, operations, and policy constraints are increasingly intertwined.
Potential 2025 U.S. tariff dynamics will stress-test ADC supply chains, elevating the value of dual sourcing, localized capabilities, and smarter procurement governance
United States tariff actions anticipated in 2025, alongside continued enforcement of trade compliance and supply-chain security expectations, are poised to influence the ADC value chain in practical, operational ways. While ADCs themselves are highly regulated therapeutic products, their production depends on globally sourced inputs, including antibodies or intermediates, highly potent small-molecule payload precursors, specialized linkers, resins and filtration materials, single-use components, and temperature-controlled packaging. Tariff adjustments affecting chemical intermediates, laboratory equipment, stainless-steel components, or certain consumables can raise landed costs and introduce procurement uncertainty even when the final drug product is domestically finished.The cumulative impact is likely to be felt most acutely in manufacturing planning and vendor strategy. ADC supply chains are multi-tiered and quality-sensitive, so switching suppliers is neither fast nor trivial due to validation requirements and change-control commitments. If tariffs elevate costs or constrain availability for specific categories of inputs, companies may respond by qualifying alternate sources earlier, negotiating longer-term contracts, and building buffers for critical materials. Over time, this can encourage partial reshoring of selected steps, especially for late-stage or commercial programs where supply continuity and audit readiness outweigh incremental cost.
Additionally, tariffs can influence partnering behavior and site-selection decisions. Sponsors may prefer development and fill-finish networks that reduce cross-border movement of sensitive intermediates, particularly highly potent payloads that require specialized containment and transport. This may shift more activity toward North American manufacturing nodes, while still relying on international innovation for discovery and early development. In that sense, the policy environment can accelerate a trend already underway: designing supply chains that are resilient, compliant, and less exposed to single-country dependency.
Importantly, the strategic response is not simply cost minimization. ADC sponsors must weigh tariff-driven cost changes against the clinical and regulatory risks of supply disruption, the timeline impact of tech transfer, and the reputational consequences of shortages in oncology care. As 2025 approaches, the most prepared organizations will treat tariffs as a scenario-planning input-integrating trade considerations into CMC roadmaps, procurement governance, and partnership structures-rather than as an after-the-fact financial adjustment.
Segmentation shows ADC success depends on aligning payload-linker design, tumor indication needs, target biology, and care delivery realities across end users and channels
Segmentation reveals that strategic priorities vary sharply depending on how antitumor ADCs are positioned clinically and operationally. When the market is viewed through the lens of product type, organizations must account for different development and manufacturing complexity across antibody components, linker technologies, and payload classes, each of which carries distinct safety, stability, and scale-up considerations. This means competitive advantage increasingly comes from mastering the interplay between these elements rather than optimizing any single component in isolation.From an indication perspective, the ADC landscape is marked by distinct clinical expectations and trial-design realities across breast cancer, lung cancer, gastric cancer, urothelial cancer, ovarian cancer, multiple myeloma, lymphoma, and other solid tumors. As programs move into earlier lines of therapy, tolerability profiles and outpatient feasibility become more decisive, pushing developers to reduce off-target toxicity and manage dose modifications with greater precision. In hematologic malignancies, where target expression and disease biology can differ substantially from solid tumors, differentiation often hinges on balancing depth of response with manageable safety signals.
Target segmentation underscores the importance of biomarker logic and resistance management. Programs focused on HER2, TROP2, Nectin-4, CD30, BCMA, CD19, and other emerging antigens must confront target heterogeneity, antigen shedding, and expression thresholds that influence both efficacy and patient selection. As a result, companies increasingly integrate companion diagnostics strategies, pursue broader “HER2-low” or analogous paradigms where biologically justified, and explore combination regimens to address resistance mechanisms.
Segmentation by technology and delivery approach highlights growing divergence between conventional conjugation methods and newer site-specific strategies designed to improve homogeneity and pharmacokinetics. Similarly, administration setting and end-user segmentation across hospitals, specialty clinics, and oncology centers influences product support models, infusion logistics, and pharmacovigilance workflows. Finally, distribution channel realities-spanning hospital pharmacies, specialty pharmacies, and tightly managed oncology supply systems-shape how manufacturers plan cold-chain controls, inventory policies, and patient access coordination. Taken together, segmentation emphasizes a clear lesson: winning strategies are those that align molecular design, clinical evidence, and delivery economics with the practical constraints of where and how ADCs are actually used.
Regional realities across the Americas, EMEA, and Asia-Pacific shape ADC adoption through distinct access rules, trial capacity, and manufacturing ecosystems
Regional dynamics in antitumor ADCs reflect differences in regulatory pathways, clinical trial infrastructure, manufacturing ecosystems, and adoption patterns across healthcare systems. In the Americas, mature oncology markets support rapid uptake for differentiated therapies, but demand robust evidence packages, strong safety monitoring, and clear value narratives that resonate with both clinicians and payers. The region’s manufacturing footprint and CDMO capabilities also influence how sponsors architect late-stage supply chains, particularly when seeking to reduce exposure to cross-border disruptions.Across Europe, Middle East & Africa, the environment is shaped by heterogeneous reimbursement models, variable speed of access, and a strong emphasis on comparative clinical value. Sponsors often need region-specific strategies that anticipate country-level health technology assessment expectations and real-world evidence requirements. Meanwhile, manufacturing and quality standards remain stringent, making compliance and traceability core to sustained commercialization. In parts of the Middle East, investment in advanced healthcare infrastructure can support faster adoption in select centers, while in other areas, access is more constrained by procurement budgets and specialized care availability.
In Asia-Pacific, growth in clinical research capacity and biopharma innovation is reshaping competitive dynamics. China, Japan, South Korea, and Australia each offer distinct advantages in trial execution, regulatory engagement, and talent pools, while regional manufacturing expansion is strengthening capabilities in biologics and increasingly in complex conjugates. As local champions deepen ADC pipelines and global companies expand partnerships, the region is becoming both a development engine and a commercialization frontier. However, market access and adoption still depend on local pricing frameworks, diagnostic availability, and the ability to support consistent quality across distributed supply networks.
Across all regions, a common theme is emerging: ADC strategies that travel well are those built on flexible evidence generation, resilient manufacturing networks, and localized engagement with oncology ecosystems. Regional insight therefore is not merely about where demand exists, but about how differences in policy, infrastructure, and clinical practice determine the fastest and safest path to sustained adoption.
Leading ADC players differentiate through platform IP, disciplined clinical evidence, and industrial-grade manufacturing execution that sustains lifecycle reliability
Company strategies in antitumor ADCs are increasingly differentiated by how they combine innovation depth with operational readiness. Large pharmaceutical organizations tend to leverage broad clinical development infrastructure, established global commercialization capabilities, and capital-intensive manufacturing investments. Their advantage often lies in running multiple pivotal programs in parallel, executing large combination trials, and scaling supply with robust quality systems.Specialized oncology and biotechnology firms, by contrast, frequently lead with focused scientific bets-novel targets, differentiated payloads, or proprietary conjugation platforms. These players often move quickly in discovery and early clinical proof-of-concept, then use partnerships to access late-stage development resources, geographic reach, or manufacturing capacity. As deal-making expands, competitive positioning is increasingly defined by the quality of platform IP, translational science credibility, and the ability to demonstrate repeatable success across multiple assets rather than a single standout candidate.
CDMOs and enabling-technology providers are also central to the competitive story, even when they are not the brand owners. Their capabilities in high-potency handling, containment, conjugation scale-up, analytical method development, and sterile fill-finish can become gating factors for timelines. As demand intensifies, sponsors are prioritizing partners with proven inspection histories, redundancy across sites, and the ability to support lifecycle changes without destabilizing supply.
Across the competitive field, a key insight is that “best molecule” is no longer sufficient. The most credible leaders pair strong clinical data with repeatable manufacturing performance, disciplined pharmacovigilance, and a clear strategy for managing class-specific safety risks. In practice, this means companies that institutionalize ADC know-how across functions-chemistry, bioanalytics, clinical operations, regulatory, and market access-are better positioned to sustain momentum as the landscape becomes more crowded.
Industry leaders can win in ADCs by integrating target-payload strategy, de-risking CMC early, modernizing trials, and building resilient partnerships
Industry leaders can strengthen their position in antitumor ADCs by acting decisively across science, operations, and access strategy. First, they should treat target selection and payload choice as an integrated decision tied to real-world tolerability, not only to mechanistic potency. Building early translational packages that connect target expression patterns, tumor microenvironment features, and expected on-target off-tumor risks helps avoid late-stage surprises and supports smarter patient selection.Second, leaders should invest earlier in CMC and supply-chain resilience. Establishing dual sourcing for critical raw materials, qualifying alternate sites for key steps, and designing processes with robust control strategies reduces vulnerability to disruptions, including those driven by changing trade conditions. Just as importantly, organizations should implement governance that links procurement decisions to quality and regulatory implications, ensuring that cost actions do not inadvertently create approval delays.
Third, companies should modernize clinical development approaches for crowded indications. Adaptive trial elements, rational combination studies, and thoughtful endpoint selection can accelerate learning while preserving credibility with regulators and clinicians. When multiple ADCs compete for similar targets, differentiation will depend on consistent safety management, convenience of administration, and durability of benefit in clinically meaningful subgroups.
Fourth, leaders should proactively shape access and evidence strategies. Aligning companion diagnostics availability, generating fit-for-purpose real-world evidence plans, and preparing clear education for infusion centers and specialty clinics can smooth adoption. Additionally, structured medical education that addresses class-related adverse events and supportive care pathways can reduce discontinuations and improve outcomes.
Finally, executives should cultivate partnership optionality. In an environment where technology, manufacturing, and geographic access needs rarely sit within one organization, a flexible partnering strategy-spanning licensing, co-development, and manufacturing alliances-can create speed advantages without sacrificing control over the most value-defining capabilities.
A triangulated methodology combining scientific literature, regulatory and trial intelligence, and expert validation links ADC science to commercial and operational realities
This research methodology integrates systematic secondary research with structured primary validation to ensure a balanced, decision-oriented view of the antitumor ADC landscape. Secondary research draws on peer-reviewed scientific literature, regulatory agency publications, clinical trial registries, company filings and investor materials, patent and intellectual property databases, conference proceedings, and credible trade and policy documentation relevant to biopharma manufacturing and supply chains. This phase establishes a foundational map of technology evolution, pipeline direction, and competitive behavior.Primary research is then used to validate assumptions and clarify real-world constraints. Interviews and expert consultations are conducted with stakeholders such as biopharma R&D leaders, clinical investigators, CMC and quality specialists, manufacturing and supply-chain executives, CDMO representatives, and market access professionals. These perspectives help interpret how scientific choices translate into operational complexity, and how regulatory and reimbursement realities influence development and commercialization strategy.
Insights are further strengthened through triangulation, where findings are cross-checked across independent sources and reconciled to resolve discrepancies. Qualitative analysis focuses on identifying consistent patterns in clinical differentiation strategies, manufacturing scale-up challenges, and regional access drivers. Throughout, the approach emphasizes practical decision support-highlighting what matters for strategy setting, risk management, and execution sequencing-rather than relying on single-source narratives.
The result is an evidence-informed synthesis designed to help leadership teams evaluate ADC opportunities with clear line-of-sight from molecular design choices to clinical positioning, operational feasibility, and region-specific adoption pathways.
ADC leaders will outperform by uniting clinical differentiation, manufacturing resilience, and region-specific access strategies into one executable playbook
Antitumor ADC drugs are entering a phase where the winners will be determined by integrated excellence rather than isolated breakthroughs. Scientific innovation remains essential, but it must be paired with operational maturity in conjugation manufacturing, high-potency handling, and end-to-end quality systems. As competition intensifies across targets and indications, developers must also elevate clinical strategy, focusing on differentiation that is meaningful to patients, clinicians, and payers.Policy and trade conditions, including the prospect of tariff-related friction in 2025, add another layer of complexity that cannot be managed reactively. Companies that embed resilience into sourcing, validation, and network design will be better positioned to maintain continuity and protect timelines. Meanwhile, regional differences in access and adoption require flexible evidence generation and localized engagement models.
Ultimately, the landscape rewards organizations that can connect the dots-from target biology and linker-payload engineering to trial execution, manufacturing robustness, and real-world delivery. Leaders that build repeatable platforms, invest early in CMC readiness, and tailor regional strategies will be best equipped to translate ADC promise into sustained oncology impact.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Antitumor ADC Drugs Market
Companies Mentioned
The key companies profiled in this Antitumor ADC Drugs market report include:- ADC Therapeutics SA
- Amgen Inc.
- Astellas Pharma Inc.
- AstraZeneca PLC
- Bayer AG
- BeiGene, Ltd.
- Daiichi Sankyo Company, Limited
- Eisai Co., Ltd.
- F. Hoffmann-La Roche Ltd
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- ImmunoGen, Inc.
- Johnson & Johnson
- Merck & Co., Inc.
- Mersana Therapeutics, Inc.
- Novartis AG
- Pfizer Inc.
- RemeGen Co., Ltd.
- Sanofi
- Seagen Inc.
- Sutro Biopharma, Inc.
- Zymeworks Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | January 2026 |
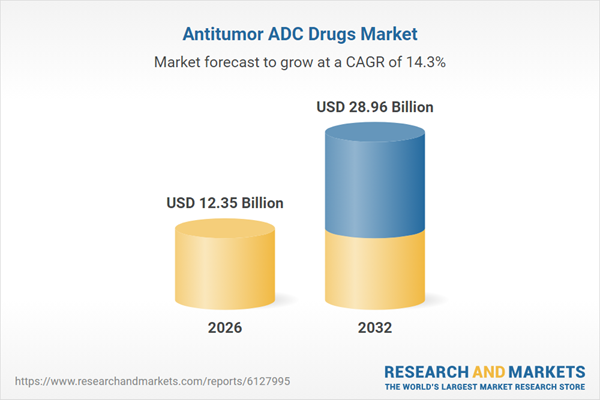
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 12.35 Billion |
| Forecasted Market Value ( USD | $ 28.96 Billion |
| Compound Annual Growth Rate | 14.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |