Speak directly to the analyst to clarify any post sales queries you may have.
PCT rapid test kits are redefining time-to-decision in infection management as stewardship priorities and workflow pressures converge across care settings
Procalcitonin (PCT) rapid test kits have become a practical bridge between the clinical need for timely infection insight and the operational reality of busy care settings. As healthcare systems intensify efforts to distinguish bacterial from viral etiologies and reduce unnecessary antibiotic exposure, PCT testing is increasingly positioned as a decision support tool that can influence triage, therapy initiation, and de-escalation. Rapid formats, in particular, align with time-sensitive pathways in emergency departments, urgent care, intensive care, and step-down units where minutes can change outcomes and resource utilization.At the same time, adoption is no longer driven solely by test availability. Clinical governance teams, antimicrobial stewardship programs, and laboratory leadership are scrutinizing how PCT results integrate with protocols, how quickly results can be returned, and how reliably assays perform across diverse patient populations. This has put renewed emphasis on operational fit, including specimen requirements, workflow compatibility, and connectivity with laboratory information systems or point-of-care data capture.
Against this backdrop, the competitive environment is evolving toward more clinically integrated solutions that pair assay performance with implementation support. Buyers are weighing total cost of ownership, training burdens, quality controls, and supply continuity as heavily as analytical characteristics. Consequently, the executive conversation has shifted from “Does the test work?” to “How does the test scale across sites, pathways, and reimbursement realities while supporting measurable stewardship goals?”
Decentralized testing, protocol-driven stewardship, and connectivity demands are transforming PCT rapid kits from products into pathway-enabling solutions
The market landscape for PCT rapid test kits is being reshaped by a set of interlocking shifts that extend beyond assay chemistry. First, stewardship has become more protocolized, with hospitals and integrated delivery networks formalizing antibiotic decision pathways that demand repeatable, auditable evidence. In this environment, PCT is increasingly evaluated as part of a broader clinical algorithm rather than a standalone biomarker, which elevates the importance of consistent result interpretation, clinician education, and pathway adherence monitoring.Second, decentralization of diagnostics continues to accelerate. Many providers are pushing testing closer to the patient to reduce turnaround time, particularly in emergency and acute care. This shift changes purchasing criteria: ease of use, minimal sample preparation, instrument uptime, and operator training requirements can outweigh marginal performance differences. In parallel, laboratories are balancing centralized quality oversight with distributed testing footprints, increasing demand for robust QC programs, remote oversight capabilities, and standardized procedures.
Third, health systems are demanding stronger interoperability and evidence of operational impact. Connectivity to electronic health records, middleware integration, and automated result routing support faster clinical action and enable analytics for stewardship reporting. As value-based care expands, buyers are also looking for vendors that can help quantify outcomes such as reduced antibiotic days, fewer admissions, or improved patient flow, even when those outcomes are influenced by broader clinical practices.
Finally, supply chain resilience has become a strategic differentiator. The experience of global disruptions has made continuity planning a formal part of vendor evaluation. Manufacturers are responding through multi-sourcing of critical components, regionalizing certain steps of production, and adjusting inventory strategies. These changes, combined with tighter quality expectations and regulatory scrutiny, are raising the bar for commercialization discipline and lifecycle management.
United States tariffs expected in 2025 may reshape PCT rapid kit sourcing and contracts, elevating supply resilience and cost transparency in procurement
The cumulative impact of United States tariffs anticipated in 2025 is poised to influence procurement strategies, pricing discipline, and supply chain design for PCT rapid test kits. Even when tariffs do not directly apply to finished diagnostic kits, upstream components such as plastics, reagents, specialized membranes, electronics, and packaging can be affected, creating indirect cost pressure. For manufacturers and distributors, these pressures can compress margins or trigger repricing discussions, especially for multi-year contracts negotiated under earlier cost assumptions.In response, many suppliers are expected to intensify supplier diversification and consider partial reconfiguration of their bills of materials. Shifting component sourcing away from tariff-exposed channels can reduce risk, but it may also introduce validation burdens, new quality audits, and potential lead-time variability. Consequently, the tariff environment can increase the strategic value of mature quality systems and flexible manufacturing networks that can qualify alternate suppliers without disrupting output.
On the buyer side, tariffs tend to accelerate a more rigorous approach to total cost of ownership. Procurement teams may push for contractual protections such as transparent escalation clauses, defined thresholds for price adjustments, and contingency commitments for allocation during disruptions. In parallel, health systems could consolidate vendors to reduce administrative complexity, favoring suppliers that can demonstrate both price stability and dependable fulfillment.
Over time, tariffs may also influence where innovation is commercialized first. If cost uncertainty is higher for certain configurations, manufacturers may prioritize platforms or kit formats that can be produced with lower exposure to tariff-sensitive components. The net effect is not simply higher costs; it is a rebalancing of risk across the value chain that rewards suppliers capable of planning, documenting, and communicating supply continuity with the same rigor applied to clinical performance.
Segmentation reveals adoption drivers across product formats, end-user workflows, and clinical applications where stewardship outcomes and speed shape kit selection
Segmentation dynamics in PCT rapid test kits reveal how adoption is shaped by practical constraints and clinical intent. Across product types, decision-makers are distinguishing between rapid tests designed for near-patient use and systems oriented to controlled laboratory environments, often aligning the choice with turnaround-time requirements and staffing models. Where fast triage is essential, decision pathways tend to favor rapid formats that reduce time between sample collection and actionable guidance, while centralized settings emphasize throughput, standardization, and tight quality management.Considering technology and assay format, competitive differentiation often stems from operational simplicity and robustness under real-world conditions. Buyers pay close attention to specimen compatibility, stability under routine storage, and performance under variable operator skill levels. As more organizations deploy PCT testing beyond a single flagship hospital, consistency across sites becomes central, increasing interest in platforms with standardized consumables and scalable training programs.
End-user segmentation further clarifies purchasing behavior. Hospitals and emergency departments typically prioritize rapid turnaround and clinical pathway integration, while diagnostic laboratories may focus on workflow efficiency, batch processing options, and quality documentation. In outpatient and urgent care environments, adoption is influenced by staffing constraints and the need for intuitive operation, especially where laboratory infrastructure is limited.
Distribution channel choices also shape market execution. Direct sales models can support complex implementations that require protocol alignment and training, whereas distributor-led routes can expand reach in fragmented markets but require disciplined channel management to maintain service quality. Finally, application-based segmentation highlights differences between sepsis evaluation, lower respiratory tract infections, and broader infection management use cases. Organizations implementing PCT primarily for antibiotic stewardship may define success through adherence and utilization metrics, while critical care teams may emphasize speed, repeat testing cadence, and interpretive support within high-acuity workflows.
Regional differences in stewardship maturity, procurement centralization, and diagnostic infrastructure shape how PCT rapid kits are evaluated and deployed at scale
Regional dynamics in PCT rapid test kits reflect differences in care pathways, procurement structures, and diagnostic infrastructure. In the Americas, stewardship programs and standardized emergency care protocols often drive structured evaluation of biomarkers, and integrated health systems may emphasize enterprise-wide harmonization of testing and reporting. As a result, suppliers that can support multi-site deployment, connectivity, and contract predictability are well positioned.Across Europe, Middle East & Africa, adoption patterns vary widely by country and health system maturity. In many European markets, strong clinical guidelines and centralized procurement can accelerate uptake once a test is aligned with pathway standards, while some regions place heightened emphasis on tender competitiveness and documented quality systems. In parts of the Middle East and Africa, growth is frequently linked to investments in hospital infrastructure and the expansion of acute care capabilities, with reliability of supply and training support emerging as critical differentiators.
In Asia-Pacific, a combination of large patient volumes, rapid expansion of healthcare capacity, and increasing attention to antimicrobial resistance is shaping demand. High-throughput urban hospitals may seek scalable solutions that can handle volume without sacrificing turnaround time, while decentralized settings value ease of use and minimal infrastructure requirements. Across the region, localization of manufacturing and distribution partnerships can be a decisive factor, particularly when import complexity or lead-time variability affects continuity.
Taken together, these regional contrasts underscore a common theme: successful commercialization depends on aligning product configuration and service models with the region’s procurement mechanics, clinical governance norms, and operational realities of diagnostic delivery.
Competitive advantage now hinges on platform integration, clinical implementation support, and resilient service models that sustain trust across health systems
Key company strategies in the PCT rapid test kit space increasingly converge on three themes: operational integration, portfolio coherence, and trust in supply continuity. Leading participants are investing in platform ecosystems that allow PCT to sit alongside complementary inflammatory and infection-related markers, enabling laboratories and care teams to standardize training, maintenance, and data workflows. This platform approach can simplify procurement decisions for buyers who prefer fewer instruments and more unified service agreements.Another defining area is implementation support. Companies that provide clear clinical use guidance, protocol templates, and education for clinicians and laboratorians can reduce adoption friction and help customers achieve consistent utilization. This is particularly important because PCT value is often realized through how results influence behavior, not merely through test availability. Vendors are therefore enhancing medical affairs capabilities and expanding post-installation support to sustain appropriate use.
Service and reliability have also become prominent competitive levers. Buyers are scrutinizing reagent availability, lot-to-lot consistency, and the ability to fulfill during demand spikes. In response, companies are strengthening quality management systems, expanding redundancy in sourcing, and improving forecasting with channel partners. Additionally, competitive differentiation is emerging through digital enablement, including connectivity tools that support result transmission, audit trails, and stewardship analytics.
Finally, partnership behavior is evolving. Collaborations with distributors, hospital networks, and technology integrators are increasingly used to accelerate reach and simplify deployment across multi-site systems. In this environment, companies that combine credible clinical positioning with disciplined operational execution are more likely to earn long-term placement in standardized care pathways.
Industry leaders can win by aligning PCT rapid kits with protocols, connectivity, resilient contracting, and sustained education that drives consistent utilization
Industry leaders can strengthen their position by treating PCT rapid test kits as part of a broader clinical and operational solution. One priority is to anchor commercialization in defined use cases with measurable workflow outcomes, such as emergency department triage or antibiotic de-escalation in respiratory infections. Aligning product messaging with protocol steps, decision thresholds, and documentation requirements helps convert interest into standardized use.Next, leaders should invest in connectivity and data utility. Making results easy to capture, route, and analyze increases clinical follow-through and enables stewardship teams to monitor compliance. Where integration is complex, offering validated interfaces, implementation playbooks, and IT support can reduce barriers that frequently delay rollouts.
Supply resilience planning should be elevated from an operations concern to a customer-facing value proposition. Proactively communicating sourcing strategies, qualification processes for alternate suppliers, and inventory approaches can reduce procurement hesitation, particularly under tariff uncertainty. Contract structures should also be revisited to reflect shared risk, balancing price stability with transparent adjustment mechanisms that preserve long-term relationships.
Finally, leaders should strengthen education and change management. Training that targets both laboratory operators and ordering clinicians improves appropriate utilization and reduces misinterpretation. Ongoing reinforcement through refresher modules, pathway audits, and clinical champions helps sustain performance over time. By coupling robust product execution with pathway-centric support, companies can improve adoption durability and deepen customer loyalty.
A rigorous mixed-method methodology combines stakeholder validation and triangulated evidence to translate PCT rapid test market complexity into decisions
The research methodology for this report is built to translate complex diagnostic markets into decision-ready insights. The approach begins with structured secondary research across regulatory documentation, public procurement signals, clinical guideline trends, peer-reviewed literature, and corporate disclosures to establish a foundational view of technology evolution, adoption drivers, and competitive positioning.Primary research then validates and enriches these findings through interviews and consultations with stakeholders across the value chain, such as laboratory leaders, clinicians involved in stewardship programs, procurement professionals, distributors, and industry executives. These discussions are used to test assumptions, clarify real-world workflow barriers, and identify how purchasing criteria are changing under constraints like staffing shortages and supply volatility.
Data triangulation is applied throughout to reduce bias and improve reliability. Insights are cross-checked across multiple perspectives and reconciled against observable market behavior, including product launches, partnership activity, and regulatory milestones. Special attention is given to ensuring that conclusions reflect practical implementation realities, not simply theoretical performance considerations.
Finally, the analysis emphasizes actionable framing. Findings are organized to support strategic decisions around product design, commercialization, channel strategy, and risk management, enabling readers to move from fragmented signals to coherent priorities grounded in industry practice.
As stewardship expectations rise, success in PCT rapid testing will depend on integrated workflows, dependable supply, and clinically grounded implementation models
PCT rapid test kits are increasingly evaluated as pathway tools that influence clinical behavior, operational efficiency, and stewardship performance. As healthcare systems seek faster, more consistent decision-making in infection management, rapid formats that align with decentralized workflows and strong quality oversight are gaining strategic relevance.Meanwhile, external pressures such as tariff-related cost uncertainty and persistent supply chain expectations are reshaping what buyers consider “value.” Reliability, transparency, and implementation support are rising alongside analytical considerations. Companies that address these priorities holistically will be better positioned to secure standardized placement across multi-site health systems.
Ultimately, the landscape is moving toward integrated solutions that combine assay access with workflow compatibility, data connectivity, and change management. Stakeholders that invest now in resilient operations and clinically grounded adoption models will be better prepared to compete as stewardship programs and procurement requirements continue to mature.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China PCT Rapid Test Kits Market
Companies Mentioned
The key companies profiled in this PCT Rapid Test Kits market report include:- Abbott Laboratories
- Bio-Rad Laboratories, Inc.
- bioMérieux SA
- Boditech Med Inc.
- Danaher Corporation
- DiaSorin S.p.A.
- EKF Diagnostics Holdings plc
- F. Hoffmann-La Roche AG
- Fujirebio Holdings, Inc.
- Getein Biotech, Inc.
- QIAGEN N.V.
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
- Trinity Biotech plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
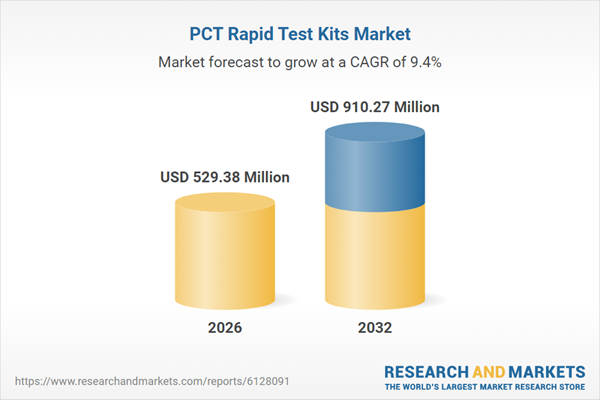
| Estimated Market Value ( USD | $ 529.38 Million |
| Forecasted Market Value ( USD | $ 910.27 Million |
| Compound Annual Growth Rate | 9.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |