Speak directly to the analyst to clarify any post sales queries you may have.
Mechanism-driven innovation and precision patient targeting are elevating melanocortin receptor agonists into a strategic focus for modern therapeutics
Melanocortin receptor agonist drugs have moved from niche scientific interest to strategic therapeutic platforms as the industry sharpens its focus on mechanistically targeted medicines. By engaging melanocortin receptors-most prominently MC4R for energy homeostasis and MC1R for melanogenesis and inflammation-these agents are increasingly associated with clinically meaningful outcomes in carefully defined patient populations. As a result, decision-makers are paying closer attention to how receptor selectivity, delivery route, and safety monitoring translate into real-world adoption and long-term differentiation.In parallel, heightened demand for precision approaches in metabolic disease, genetic obesity syndromes, dermatology, and inflammatory indications is reshaping how sponsors prioritize development programs. The bar for evidence is rising as payers and clinicians expect durable efficacy, clean tolerability, and practical administration. Consequently, stakeholders across R&D, manufacturing, market access, and commercialization are converging on a central question: which melanocortin agonist strategies can deliver patient-relevant benefit while remaining operationally scalable and economically defensible.
This executive summary frames the current environment through the lenses that matter most for leaders: the forces transforming competition and access, the evolving trade and tariff context, the segmentation patterns that influence clinical adoption, the regional nuances in regulation and care delivery, and the company strategies shaping differentiation. Together, these insights support clearer choices on portfolio direction, partnering, and go-to-market readiness.
Receptor-selective development, tougher evidence standards, delivery-centric differentiation, and supply resilience are redefining competition and adoption dynamics
The landscape for melanocortin receptor agonist drugs is undergoing structural change as science, regulation, and commercialization converge around tighter definitions of value. One major shift is the transition from broad mechanistic claims to receptor- and pathway-specific product narratives. Developers are moving beyond “melanocortin activation” as a monolithic concept and increasingly emphasize receptor subtype selectivity, tissue targeting, and the downstream physiologic effect that best aligns with an indication’s unmet need. This has made translational biomarkers and genotype-informed patient identification more central to clinical development and post-approval adoption.At the same time, regulatory expectations are evolving in ways that reward rigorous long-term evidence and penalize ambiguity in benefit-risk. For agents acting on pathways tied to appetite, autonomic tone, pigmentation, and immune modulation, safety scrutiny extends beyond typical adverse events to include cardiovascular parameters, psychiatric signals, and off-target endocrine effects where biologically plausible. Consequently, companies are adapting trial design with longer follow-up, more comprehensive monitoring, and pragmatic endpoints that anticipate payer and clinician questions.
Another transformative shift is the heightened importance of delivery innovation and adherence economics. Where chronic therapy is expected, the convenience and tolerability of administration increasingly determine persistence, and persistence increasingly determines realized effectiveness. Sponsors are therefore balancing potency with usability, including device considerations, titration strategies, and patient support services that reduce discontinuation risk. In parallel, the competitive set is no longer limited to direct melanocortin peers; it includes adjacent metabolic and dermatology innovations that can displace demand unless melanocortin agonists demonstrate clear differentiation.
Finally, manufacturing resilience and supply continuity have become strategic differentiators rather than back-office concerns. Peptide-based or complex synthetic modalities require tight control of quality attributes, and minor disruptions can cascade into access constraints. As industry leaders re-evaluate single-source dependencies and international supply exposure, the ability to assure consistent quality, redundant capacity, and predictable delivery timelines is now integral to the product value story.
Potential 2025 U.S. tariff pressures are pushing melanocortin agonist programs toward multi-sourcing, contract redesign, and more resilient end-to-end supply models
United States tariffs anticipated in 2025 are poised to reshape procurement decisions for pharmaceutical inputs, manufacturing equipment, and selected finished goods, with practical implications for melanocortin receptor agonist programs. Even when finished drug products are not directly targeted, the cost and availability of upstream materials-such as peptide synthesis reagents, specialized excipients, single-use manufacturing components, and packaging inputs-can be affected through indirect exposure. As a result, finance and operations teams are reassessing total landed cost and the stability of supplier networks rather than focusing solely on list pricing.In response, companies are likely to accelerate multi-sourcing and regionalization strategies for critical inputs, particularly where qualification timelines are long. For complex peptides and high-specification materials, supplier changes require substantial comparability work, quality agreements, and validation. Therefore, the tariff environment encourages earlier investment in secondary suppliers and in-house analytical capabilities to reduce switching risk. This shift can benefit sponsors that already have mature quality systems and supplier governance, while placing smaller organizations under pressure to secure manufacturing partners with robust procurement leverage.
Tariff-related uncertainty also influences contracting and inventory approaches. Some manufacturers may increase safety stocks for vulnerable inputs, but that can strain cash flow and warehousing capacity, especially for materials with limited shelf life. In parallel, contract terms are likely to evolve to include clearer pass-through clauses, revised incoterms, and shared risk mechanisms. Commercially, higher input volatility may affect net pricing discussions as payers scrutinize price changes that appear cost-driven rather than value-driven.
Over time, the cumulative impact is expected to reward organizations that treat trade policy as an enterprise risk discipline. By integrating scenario planning into supply chain design, aligning regulatory strategy with supplier flexibility, and coordinating with partners on tariff exposure mapping, leaders can reduce disruption risk and protect launch timelines. Conversely, programs that underestimate the operational footprint of tariffs may experience avoidable delays, budget overruns, or constrained supply during critical adoption windows.
Segmentation dynamics show that receptor subtype, indication priorities, administration practicality, and channel execution jointly determine real-world melanocortin agonist uptake
Segmentation patterns reveal that melanocortin receptor agonist adoption is shaped by the intersection of receptor subtype focus, therapeutic intent, and practical delivery constraints. Across receptor targeting, MC4R-oriented strategies continue to concentrate attention where energy balance and satiety signaling are central, whereas MC1R-focused approaches align more naturally with pigmentation biology and selected inflammatory pathways. This mechanistic split affects everything from endpoint selection to specialist ownership, and it directly influences how evidence is packaged for payers and clinical guideline bodies.From the standpoint of therapeutic area, obesity-especially genetically defined or syndromic forms-rewards precision positioning because clinicians seek confidence that responders can be identified and monitored effectively. Dermatology use cases, by contrast, often require a distinct risk-benefit conversation rooted in visible outcomes, patient expectations, and longer-term safety considerations related to pigmentation changes. Meanwhile, emerging interest in inflammatory and immunomodulatory applications raises the bar for demonstrating pathway relevance and avoiding off-target effects, particularly where established biologics already set high expectations for efficacy.
Route of administration and dosage form meaningfully shape real-world persistence. Where injectables remain necessary, program success depends on patient education, tolerability management, and device usability, as well as careful titration strategies that reduce early discontinuation. Oral approaches, when technically feasible, can widen addressability but must balance bioavailability, food effects, and consistent exposure to avoid variability that undermines physician confidence. Long-acting formulations introduce another set of trade-offs by improving convenience while increasing the need for predictable pharmacokinetics and robust safety monitoring.
Distribution channel and care setting segmentation further differentiate commercialization playbooks. Hospital and specialty clinic pathways often prioritize clear protocols and coordination with diagnostic services, which can support genotype-informed prescribing where relevant. Retail and specialty pharmacy dynamics emphasize prior authorization navigation, patient support infrastructure, and adherence reinforcement. Ultimately, the winning segmentation strategy ties mechanistic clarity to operational simplicity, ensuring that the right patients are identified, the therapy is practical to use, and the value narrative remains consistent from prescriber to payer to patient.
Regional adoption hinges on regulatory nuance, payer thresholds, and care infrastructure across the Americas, Europe Middle East & Africa, and Asia-Pacific ecosystems
Regional dynamics for melanocortin receptor agonist drugs reflect differences in regulatory interpretation, access thresholds, and health system capacity to support targeted prescribing. In the Americas, clinical adoption often hinges on strong evidence packages that address long-term outcomes and safety monitoring, while payer scrutiny drives demand for clear responder identification and structured patient support. This environment can favor products with well-defined labeling, strong real-world evidence plans, and scalable specialty pharmacy partnerships.Across Europe, Middle East & Africa, heterogeneous reimbursement systems and country-by-country health technology assessment approaches amplify the importance of localized value narratives. Clinical guidelines, prescribing restrictions, and center-of-excellence models can accelerate uptake for narrowly defined populations, yet they can also slow expansion beyond initial subgroups unless manufacturers invest in physician education and region-specific evidence generation. Moreover, supply reliability and consistent quality documentation play an outsized role where parallel trade dynamics and tendering processes influence procurement decisions.
In Asia-Pacific, growth in specialty care infrastructure and expanding diagnostic capabilities are improving readiness for targeted therapies, but access pathways vary widely between mature and emerging markets. Regulatory agencies increasingly expect global-quality clinical and manufacturing data, while local partnership models can determine speed to distribution and formulary inclusion. Patient affordability and the evolution of insurance coverage remain pivotal, making differentiated patient access programs and pragmatic care pathways essential to sustained adoption.
Across all regions, the most durable strategies align regulatory engagement, medical education, and supply planning with the realities of local care delivery. Companies that treat regionalization as a core design principle-rather than a late-stage commercialization task-are better positioned to scale responsibly and protect brand credibility.
Company differentiation now hinges on translating melanocortin biology into defensible clinical value, scalable operations, and partnership-led execution across indications
Competitive positioning among melanocortin receptor agonist developers increasingly depends on how each company converts receptor biology into a coherent clinical and commercial narrative. Established pharmaceutical organizations tend to emphasize integrated capabilities, including rigorous safety surveillance, manufacturing scale, and global access execution. These strengths can be decisive for therapies that require careful monitoring, specialized distribution, or broad physician education to ensure appropriate use.Specialty biopharma companies and innovation-led developers often differentiate through sharper mechanistic focus, faster iteration on formulation or device design, and deep engagement with patient communities. In rare genetic obesity settings, for example, success is frequently tied to patient identification ecosystems and the ability to partner with centers that diagnose and manage complex disease. These companies may also prioritize evidence generation models that quickly demonstrate functional improvement and quality-of-life benefits that matter to patients and caregivers.
Across the company landscape, partnering behavior remains a defining feature. Co-development, regional licensing, and manufacturing alliances help spread risk and accelerate execution, particularly when supply chains rely on specialized peptide capabilities or when commercial infrastructure must be built quickly. Meanwhile, competitive intensity is rising from adjacent mechanisms in metabolic disease and dermatology, pushing melanocortin agonist sponsors to sharpen differentiation around durability, tolerability, and ease of use.
Looking ahead, the most credible company strategies will pair scientific specificity with operational excellence. Organizations that can defend receptor selectivity, manage class-related safety questions transparently, and deliver uninterrupted supply are best positioned to earn clinician trust and payer confidence over multiple product life-cycle stages.
Leaders can win by aligning evidence, access, and operations - prioritizing persistence, resilient supply, segmentation-led commercialization, and capability-filling partnerships
Industry leaders can strengthen their position by designing development programs that anticipate access questions rather than reacting to them. This starts with evidence strategies that clearly link receptor subtype selectivity to patient-relevant outcomes, supported by fit-for-purpose biomarkers and practical monitoring protocols. When the indication involves chronic therapy, leaders should treat persistence as a core endpoint proxy, building tolerability management and patient support into both trial operations and launch planning.Given the operational risks highlighted by evolving trade policy, leaders should also elevate supply chain resilience to a board-level priority. Dual sourcing for critical inputs, proactive supplier qualification, and robust comparability plans can reduce disruption risk. In parallel, contracts with manufacturing and distribution partners should explicitly address volatility, including change-control governance and contingency capacity, to protect both launch timing and ongoing availability.
Commercially, a segmentation-aligned go-to-market model is essential. Companies should align field education to the specialist owners of each indication, ensure diagnostic and referral pathways are practical, and invest in reimbursement tools that reduce administrative burden. Where therapy requires careful patient selection, leaders can enable adoption by supporting testing workflows and by providing clear clinical decision frameworks that physicians can apply without friction.
Finally, leaders should pursue partnerships that fill capability gaps rather than chasing scale for its own sake. Whether the need is specialty distribution, device engineering, regional market access, or peptide manufacturing depth, targeted alliances can accelerate readiness while keeping strategic control of the value narrative. The overarching goal is to make melanocortin receptor agonist therapies straightforward to prescribe, straightforward to supply, and straightforward to justify.
A triangulated methodology combining scientific, regulatory, and stakeholder inputs clarifies development realities, adoption barriers, and operational risks for leaders
This research methodology integrates structured secondary research with targeted primary validation to capture how melanocortin receptor agonist drugs are developing across science, regulation, and commercialization. Secondary research draws from peer-reviewed literature, clinical trial registries, regulatory agency documentation, corporate disclosures, patent filings, and scientific conference materials to map mechanisms, development status, and product positioning. These sources inform an initial framework for understanding how receptor subtype targeting and formulation choices align with therapeutic priorities.Primary research is conducted through interviews and consultations with stakeholders across the ecosystem, including clinicians, pharmacy and access experts, manufacturing and quality leaders, and industry executives. These discussions validate practical adoption drivers such as patient identification workflows, monitoring expectations, persistence challenges, and reimbursement frictions. They also help clarify how procurement and supply decisions are evolving in response to policy shifts, including tariff-related concerns.
Triangulation is used throughout to reconcile discrepancies across sources and to ensure that conclusions are grounded in consistent signals rather than isolated opinions. The analysis emphasizes qualitative and strategic insights, focusing on how stakeholders make decisions, where bottlenecks occur, and which capabilities create competitive advantage. Assumptions are explicitly tested through cross-checking across multiple evidence types.
Finally, the deliverable is structured to support executive decision-making. Findings are organized around segmentation and regional realities, competitive positioning, and actionable implications for development, supply, and commercialization, enabling readers to translate insights into concrete priorities.
Melanocortin agonist success will be defined by receptor-specific proof, patient-centric usability, resilient supply, and region-ready access execution
Melanocortin receptor agonist drugs are entering a more disciplined era in which mechanistic promise must be matched by practical deliverability. The most successful programs will be those that articulate receptor-specific value, prove durable patient benefit, and address predictable safety and monitoring expectations. As adjacent therapeutic innovations intensify competition, differentiation will depend as much on usability and persistence as on pharmacology.Operational resilience is equally decisive. With supply chains exposed to policy shifts and specialized input dependencies, organizations must design manufacturing and procurement strategies that withstand disruption. Regional complexity further reinforces the need for localized access narratives and tailored execution models that reflect how care is delivered and reimbursed.
Taken together, the outlook rewards companies that connect science to systems. By integrating evidence strategy, supply planning, and commercialization design early, leaders can reduce uncertainty, accelerate responsible adoption, and build durable trust with clinicians, payers, and patients.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Melanocortin Receptor Agonist Drugs Market
Companies Mentioned
The key companies profiled in this Melanocortin Receptor Agonist Drugs market report include:- AMAG Pharmaceuticals, Inc.
- Catalent
- Clinuvel Pharmaceuticals Ltd.
- Crinetics Pharmaceuticals
- LG Chem
- Palatin Technologies, Inc.
- Rhythm Pharmaceuticals, Inc.
- SynAct Pharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
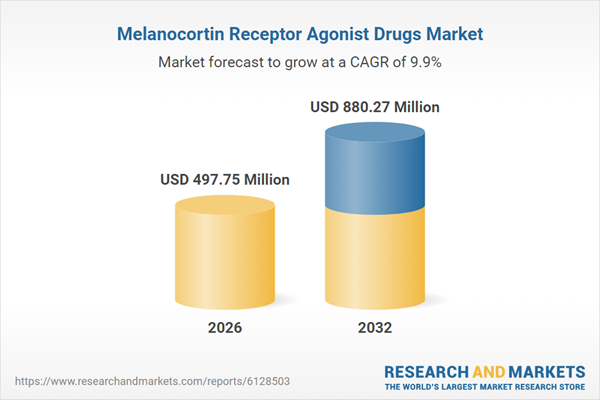
| Estimated Market Value ( USD | $ 497.75 Million |
| Forecasted Market Value ( USD | $ 880.27 Million |
| Compound Annual Growth Rate | 9.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |