Speak directly to the analyst to clarify any post sales queries you may have.
CAR-T development services are becoming the decisive engine that converts ambitious immunotherapy concepts into regulated, reproducible, and scalable products
CAR-T therapies have evolved from breakthrough science into a demanding industrial discipline where execution quality is inseparable from clinical ambition. Development services now sit at the center of this transition, translating novel constructs into reproducible, compliant products while protecting timelines that are often constrained by patient need and competitive intensity. As a result, sponsors increasingly treat the development pathway as a strategic system rather than a set of isolated handoffs, with early choices in vector design, analytics, and process architecture reverberating through IND-enabling work, clinical manufacturing, and eventual commercial readiness.What makes CAR-T uniquely complex is the convergence of living-cell variability, tight chain-of-identity requirements, and an accelerating pace of innovation in gene delivery and cell engineering. Autologous models demand resilience against manufacturing failures and logistics interruptions, while allogeneic approaches introduce new requirements for donor management, gene editing control strategies, and comparability. In both cases, regulators expect deeper product understanding, more rigorous control of critical quality attributes, and clearer rationales for specifications and release strategies.
Against this backdrop, CAR-T cell development service providers are not merely vendors; they operate as technical co-architects of the product. Their capabilities in process development, analytical method development, viral vector strategy, and quality systems shape the speed of iteration and the credibility of the CMC narrative. Consequently, the market is being defined by integrated offerings, automation readiness, and an ability to scale without sacrificing patient-level traceability or product consistency.
Industrialization, integration, and modality diversification are redefining CAR-T development services as sponsors demand faster iteration with stronger CMC credibility
The most significant shift in the CAR-T development services landscape is the move from fragmented outsourcing to integrated, platform-oriented collaboration. Sponsors increasingly prefer partners that can connect early discovery support with cell processing development, viral vector readiness, analytical characterization, and GMP manufacturing, because each interface between organizations can create data discontinuities and comparability risks. This has elevated providers that can run end-to-end development while also supporting tech transfer in a way that preserves process intent and analytical continuity.In parallel, the sector is transitioning from artisanal processes toward industrialized workflows. Closed and automated systems are no longer viewed as optional efficiency upgrades; they are becoming core enablers for consistency, operator safety, contamination control, and scalable throughput. The push for digital chain-of-identity, electronic batch records, and in-process monitoring reflects a broader expectation that CAR-T will mature into a data-rich manufacturing discipline. Development service teams are investing in process analytical technology, standardized work instructions, and fit-for-purpose facility designs that reduce variability while supporting rapid change control.
Another transformative change is the expansion of modality diversity within CAR-T programs. Beyond conventional second-generation constructs, developers are pursuing armored CARs, dual-targeting strategies, logic-gated approaches, and gene-edited cell products intended to improve persistence and reduce exhaustion. These innovations increase analytical and regulatory complexity, particularly around vector copy number, insertional risk considerations, gene editing outcomes, and potency characterization. As a result, development service providers are differentiating through advanced analytical platforms, deeper bioassay expertise, and statistical approaches that support assay lifecycle management.
Finally, competitive dynamics are shifting as capacity alone becomes insufficient. Providers that once relied on “available slots” now face sponsor expectations for proactive risk management, supply chain transparency, and regulatory-grade documentation from the earliest stages. In this environment, credibility is built through demonstrated comparability strategies, robust deviation handling, and an ability to scale globally while maintaining harmonized quality systems.
United States tariffs in 2025 are set to pressure CAR-T supply chains, forcing new sourcing, validation, and location strategies across development services
United States tariff actions taking effect or escalating in 2025 are poised to reshape procurement strategies across the CAR-T development services ecosystem, particularly for imported single-use components, certain laboratory instruments, and upstream materials with global supply chains. Even when final cell processing occurs domestically, many critical inputs originate abroad, which can increase landed costs, complicate budgeting, and lengthen lead times. For sponsors, these pressures arrive at a moment when programs already face tight manufacturing windows and limited tolerance for interruptions.The cumulative impact is most visible in three operational domains. First, procurement teams must reassess supplier diversification for consumables such as bags, tubing assemblies, filters, and connectors that are essential to closed-system processing. Second, analytical laboratories may encounter higher costs or longer delivery cycles for specialized equipment and replacement parts, affecting method development throughput and validation schedules. Third, cold-chain and specialty logistics can be indirectly affected as packaging materials and monitoring devices become more expensive or harder to source on predictable timelines.
In response, development service providers are likely to strengthen dual-sourcing strategies, increase safety stock for high-risk items, and prioritize design choices that reduce dependency on tariff-exposed components. This may accelerate interest in domestically manufactured single-use assemblies, alternative vendors that can meet biocompatibility and extractables/leachables requirements, and modular equipment strategies that allow for faster substitution without undermining validation status. However, these mitigations introduce their own complexities, including the need for comparability assessments, supplier qualification work, and documentation updates that can ripple into regulatory submissions.
Strategically, tariffs can also influence where sponsors place development and manufacturing work. While reshoring may appear attractive, real-world constraints-such as the availability of trained operators, qualified raw material supply, and validated capacity-mean that decisions will hinge on total risk, not just unit cost. Sponsors that treat tariffs as a catalyst to modernize supply chain governance, standardize components across programs, and embed flexibility into their CMC plans will be better positioned to maintain momentum through 2025 and beyond.
Segmentation patterns reveal how sponsors choose CAR-T development services by stage, modality, vector strategy, and engagement model to manage risk and speed
Key segmentation dynamics in CAR-T cell development services reflect how sponsors balance scientific ambition with operational realism. Across service type, demand is increasingly concentrated in offerings that reduce handoffs and compress timelines, particularly when process development, analytical development, and GMP readiness are coordinated under a single technical governance model. At the same time, sponsors continue to unbundle select tasks when they require highly specialized expertise, such as advanced potency bioassays or vector characterization, creating a hybrid outsourcing pattern where integrated providers must still collaborate seamlessly with niche laboratories.Differences by development stage shape both scope and urgency. Early-stage programs emphasize rapid feasibility, vector selection, and fit-for-purpose analytics that can evolve without forcing revalidation too soon. As programs approach IND-enabling milestones, the emphasis shifts toward robustness studies, control strategy definition, and method qualification that can withstand regulatory scrutiny. Later-stage and commercialization-oriented work elevates comparability, continued process verification readiness, and lifecycle management, pushing service providers to demonstrate mature quality systems and disciplined change control.
Segmentation by product type and technology approach continues to widen. Autologous CAR-T relies on vein-to-vein execution excellence, where process consistency must be achieved despite patient-specific variability and scheduling constraints. Allogeneic CAR-T shifts complexity toward donor qualification, cell banking strategy, and tighter oversight of gene editing outcomes, while also increasing the need for scalable, high-throughput process design. These differences influence the development service mix, with allogeneic programs often demanding deeper platform standardization and stronger analytics for identity, purity, and residuals.
Vector strategy remains a central axis of segmentation, because it dictates timelines, cost drivers, and CMC risk. Lentiviral and retroviral systems require robust control of replication-competent virus testing, vector copy number assessment, and supplier qualification. Non-viral approaches may reduce certain risks but introduce others, including delivery efficiency, editing consistency, and potentially more complex potency interpretation. Consequently, sponsors segment provider evaluations by demonstrated mastery of vector-related analytics, tech transfer track record, and the ability to align vector supply with cell manufacturing schedules.
Finally, end-user and engagement model segmentation is shaping buying behavior. Emerging biotechs often prioritize speed, packaged expertise, and flexible contracting that matches financing milestones, while larger biopharma organizations emphasize governance rigor, global quality alignment, and long-term scalability. Across both groups, the preference is shifting toward partners who can transparently articulate tradeoffs, provide decision-grade data quickly, and manage the interplay between process development and analytical strategy without creating downstream comparability surprises.
Regional execution realities across the Americas, Europe, Middle East & Africa, and Asia-Pacific shape CAR-T development services beyond cost or capacity
Regional dynamics in CAR-T development services are increasingly shaped by talent density, regulatory expectations, and the maturity of supporting supply chains. In the Americas, demand is driven by active clinical development ecosystems, established quality frameworks, and a growing emphasis on domestic resilience for critical materials and capabilities. Providers differentiate through integrated offerings, deep regulatory experience, and the ability to support multi-site execution while maintaining harmonized documentation and data practices.Across Europe, the landscape reflects both strong scientific hubs and a complex regulatory and reimbursement environment that influences development choices. Sponsors often prioritize partners that can navigate cross-border logistics, multilingual documentation realities, and evolving expectations for advanced therapy medicinal products. The region’s emphasis on robust quality systems and comparability planning aligns with the growing need for structured analytical strategies and standardized manufacturing workflows.
In the Middle East and Africa, the market is comparatively earlier in its development but gaining momentum through targeted investments in healthcare infrastructure and specialized centers. For development services, progress often depends on building skilled workforces, establishing reliable cold-chain capabilities, and enabling compliant importation of critical materials. Partnerships with experienced global providers and structured knowledge transfer programs are important mechanisms for accelerating readiness while maintaining patient safety and regulatory compliance.
The Asia-Pacific region combines rapid capacity expansion with increasing technical sophistication. Established biopharma and emerging innovators alike are investing in cell therapy infrastructure, and sponsors often look for providers that can balance speed with stringent quality expectations. This region’s growth is also linked to improvements in local supply chains for consumables and reagents, which can reduce lead times and strengthen resilience. As cross-regional trials expand, providers that can support harmonized data packages and comparable manufacturing approaches across geographies will have an advantage.
Taken together, regional insights underscore that location decisions are no longer based solely on capacity or cost. Sponsors increasingly assess the full ecosystem-talent, materials access, regulatory navigation, and logistics robustness-because CAR-T programs succeed when development service execution is resilient across the entire value chain.
Leading CAR-T development service companies stand out through integrated execution, advanced analytics, resilient supply chains, and governance that prevents rework
Key companies in CAR-T cell development services are differentiating through breadth of integration, depth of analytics, and the maturity of their quality and supply chain systems. Providers with end-to-end capabilities can reduce friction between process development, method development, and GMP execution, which is especially valuable when rapid iteration is required and comparability risk must be tightly controlled. However, leadership is not defined solely by scale; it also depends on the ability to consistently deliver right-first-time documentation, transparent deviations management, and realistic scheduling grounded in supply chain constraints.A major axis of competition is analytical sophistication. As potency expectations intensify and products become more complex, service providers are investing in cell-based functional assays, multi-parameter flow cytometry, molecular methods, and stability strategies tailored to living-cell products. Companies that can translate analytical data into a coherent control strategy, with defensible links between critical quality attributes and clinical performance hypotheses, are increasingly trusted as long-term partners rather than transactional vendors.
Another differentiator is vector and raw material strategy, including relationships with qualified suppliers and the ability to coordinate release testing and delivery windows with manufacturing schedules. Providers that can anticipate bottlenecks, qualify alternatives, and document changes with regulatory discipline help sponsors avoid delays that are otherwise easy to trigger in autologous programs. In addition, readiness for automation and digital quality systems is emerging as a clear signal of operational maturity, particularly where chain-of-identity and data integrity must be maintained across complex workflows.
Finally, companies are competing on collaboration models. Sponsors value governance structures that enable fast decisions, clear escalation paths, and shared ownership of risk. Providers that bring program management rigor, cross-functional technical leadership, and pragmatic regulatory insight can materially reduce rework and shorten the path from development to clinical delivery.
Actionable priorities for CAR-T leaders include system-level CMC planning, accountable partnering, early dual-sourcing, and data-driven automation readiness
Industry leaders can strengthen CAR-T development outcomes by treating CMC strategy as a living system that is continuously de-risked rather than “locked” too early. This starts with aligning target product profiles to manufacturing realities, explicitly defining which attributes must be tightly controlled now versus which can be progressively refined as clinical evidence grows. By doing so, organizations can prioritize experiments that reduce the highest-impact uncertainties in potency, identity, and process robustness.Partnering strategy should be redesigned around interfaces and accountability. When using multiple providers, leaders should implement a single cross-company technical governance cadence that owns comparability and change control decisions, with shared data standards and a clearly defined “source of truth” for batch records and method versions. Conversely, when selecting an integrated provider, leaders should require transparency into subcontracting, raw material sourcing, and contingency planning so that integration does not become a black box.
Given the pressures introduced by tariffs and broader supply chain volatility, leaders should institutionalize component rationalization and dual-sourcing earlier than has been typical in cell therapy. This includes qualifying alternative consumables where feasible, using standardized single-use architectures, and maintaining a proactive obsolescence plan for instruments and software that underpin analytics. Importantly, these actions must be tied to a comparability framework so substitutions do not trigger avoidable regulatory friction.
Finally, organizations should invest in data and automation readiness as strategic capabilities. Closed-system workflows, digital chain-of-identity, and robust data governance reduce deviation rates and accelerate investigations when issues occur. Over time, these capabilities also support faster tech transfer and easier global expansion, because well-structured data and standardized processes travel better than artisanal know-how.
A structured research methodology combines primary stakeholder input with validated public evidence to produce decision-ready CAR-T development services insights
This research applies a structured methodology designed to translate complex technical markets into decision-ready insights for sponsors, service providers, and investors. The approach begins with a clear definition of the CAR-T cell development services scope, mapping activities across development stages and clarifying how services relate to adjacent domains such as viral vector supply, analytical testing, and GMP manufacturing interfaces. This scoping step is essential to avoid category confusion and to ensure that comparisons are made on like-for-like capabilities.Primary research is conducted through structured interactions with industry participants, including leadership and technical stakeholders involved in process development, analytical development, manufacturing operations, quality, and supply chain management. These engagements focus on current execution challenges, evolving sponsor expectations, technology adoption patterns, and sourcing strategies under changing regulatory and geopolitical conditions. Insights are captured in a consistent framework to enable cross-validation and to distinguish widely observed patterns from organization-specific practices.
Secondary research complements these inputs through review of publicly available materials such as regulatory guidance, company disclosures, scientific publications, conference materials, and patent and clinical trial registries where appropriate. This evidence base is used to triangulate claims, track technology and workflow trends, and contextualize how policy shifts-such as tariffs-can influence procurement and operational decisions. Throughout the process, emphasis is placed on factual consistency, practical relevance, and avoidance of unsupported inference.
Finally, findings are synthesized into segmentation and regional perspectives that help readers connect strategic choices to operational consequences. The output is designed to support partner evaluation, internal capability planning, and risk management by presenting clear narratives, traceable logic, and actionable implications rather than relying on generic descriptions of the sector.
CAR-T development services now reward disciplined execution, resilient supply chains, and scalable analytics that keep innovation moving without losing control
CAR-T cell development services are entering a phase where operational excellence and strategic adaptability determine success as much as scientific novelty. Integrated development models, industrialized workflows, and advanced analytics are becoming standard expectations, while modality innovation and stricter regulatory scrutiny continue to raise the bar for CMC credibility. In this environment, sponsors that treat development services as core partners-rather than interchangeable capacity-can materially improve cycle times and reduce avoidable comparability and quality risks.Meanwhile, the cumulative effects of supply chain volatility and the 2025 tariff environment reinforce the need for resilient sourcing strategies, earlier qualification of alternatives, and tighter alignment between procurement decisions and regulatory documentation. Regional differences further highlight that execution depends on the surrounding ecosystem, including talent availability, logistics reliability, and quality maturity.
The path forward is clear: organizations that invest in disciplined governance, data integrity, and scalable process design will be better positioned to advance programs efficiently and withstand external shocks. By aligning technical choices with operational realities, stakeholders can convert CAR-T’s clinical promise into more predictable development and delivery outcomes.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China CAR-T Cell Development Service Market
Companies Mentioned
The key companies profiled in this CAR-T Cell Development Service market report include:- Allogene Therapeutics, Inc.
- Anixa Biosciences, Inc.
- Arcellx, Inc.
- Atara Biotherapeutics, Inc.
- Aurora Biopharma, Inc.
- Autolus Therapeutics plc
- Beam Therapeutics Inc.
- BioNTech SE
- bluebird bio, Inc.
- Bristol-Myers Squibb Company
- Cellectis S.A.
- CRISPR Therapeutics AG
- Gilead Sciences, Inc.
- ImmunoACT Private Limited
- Johnson & Johnson
- Miltenyi Biotec B.V. & Co. KG
- Novartis AG
- Rocket Pharmaceuticals, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
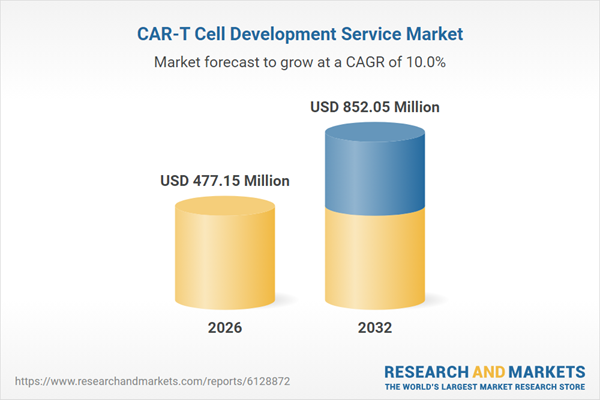
| Estimated Market Value ( USD | $ 477.15 Million |
| Forecasted Market Value ( USD | $ 852.05 Million |
| Compound Annual Growth Rate | 10.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 19 |