Speak directly to the analyst to clarify any post sales queries you may have.
Ceftazidime Dihydrochloride remains a critical hospital antibiotic where clinical urgency, quality assurance, and resilient supply chains converge
Ceftazidime dihydrochloride sits at the intersection of acute-care necessity and industrial precision. As a third-generation cephalosporin commonly positioned for serious gram-negative infections, it remains an essential tool in hospital formularies, particularly when clinicians need dependable coverage against organisms such as Pseudomonas aeruginosa. Its continued relevance is reinforced by persistent burdens of hospital-acquired infections, expanding critical-care capacity in many health systems, and ongoing stewardship efforts that aim to preserve efficacy without compromising timely access.From a market and supply-chain standpoint, ceftazidime dihydrochloride is not simply a commodity antibiotic. It is a sterile-leaning, quality-sensitive product family where manufacturing discipline, impurity control, and reliable cold-chain-adjacent logistics can strongly influence purchase decisions. Buyers increasingly evaluate continuity of supply, audit readiness, and documentation maturity alongside price, especially as drug shortages and quality-related disruptions have drawn heightened scrutiny.
Against this backdrop, stakeholders across manufacturers, distributors, group purchasing organizations, and hospital pharmacies are reassessing what “resilience” means for established injectable and hospital antibiotics. The executive summary that follows highlights the structural changes reshaping the landscape, the implications of updated trade measures, and the segmentation and regional patterns that inform competitive positioning and operational planning.
Shifting stewardship priorities, tougher quality expectations, and supply-chain resilience strategies are redefining competition for ceftazidime dihydrochloride
The landscape for ceftazidime dihydrochloride is being reshaped by a combination of clinical practice shifts and manufacturing realities. First, antimicrobial stewardship programs continue to mature, and they are no longer limited to simple restriction policies. Many systems now use rapid diagnostics, local antibiograms, and guideline-driven pathways to narrow therapy sooner. This changes demand patterns from broad, routine use to more protocolized utilization, often concentrating volumes in high-acuity settings and in institutions with strong infectious-disease governance.At the same time, competitive dynamics are evolving as procurement functions intensify supplier qualification requirements. Health systems increasingly prioritize vendors that can demonstrate redundant manufacturing, stable access to key starting materials, and rapid response to quality events. This is a meaningful shift away from purely price-led decisions and toward total-cost and risk-weighted procurement, particularly for injectable antibiotics that have historically been vulnerable to shortage cycles.
Regulatory expectations are also exerting a transformative influence. Sterile and semi-sterile manufacturing controls, data integrity standards, and increased attention to nitrosamine and related impurity risks across pharmaceuticals have sharpened focus on analytical methods, change control, and supplier oversight. Even when ceftazidime dihydrochloride itself is not a headline impurity case, organizations are applying broader risk frameworks that raise the compliance bar for legacy products.
Finally, the push toward supply localization and “friend-shoring” is changing how stakeholders think about capacity placement. Rather than assuming a single low-cost region can reliably serve global needs, manufacturers and buyers are examining dual-sourcing, regional packaging, and local release testing to reduce lead times and limit exposure to cross-border shocks. Together, these shifts are transforming ceftazidime dihydrochloride from a steady-state hospital antibiotic into a product category where operational excellence and compliance posture increasingly define competitive advantage.
United States tariffs in 2025 intensify landed-cost uncertainty and accelerate supplier diversification and compliance-driven supply-chain redesign for ceftazidime
The introduction and expansion of United States tariffs in 2025 adds a new layer of complexity for ceftazidime dihydrochloride supply chains that already operate under tight quality and lead-time constraints. Although tariff applicability depends on the specific classification of inputs and finished goods, the practical effect for many stakeholders is increased landed-cost uncertainty and a stronger incentive to map exposure across active pharmaceutical ingredient sources, intermediates, excipients, packaging, and contract manufacturing steps.In the near term, tariffs can amplify procurement volatility. Buyers may pull forward orders to lock in pricing or build safety stock, while manufacturers may re-route sourcing to alternate countries, negotiate revised Incoterms, or shift final packaging and labeling steps to reduce duty impacts where feasible. These adjustments can strain capacity planning and increase the operational load on quality teams, because even modest supplier changes often trigger audits, comparability assessments, and updated regulatory documentation.
Over the medium term, the tariff environment tends to reward organizations that have already invested in optionality. Companies with qualified secondary suppliers, regionally diversified production networks, and robust change-control systems can adapt with fewer service disruptions. Conversely, suppliers that rely on single-region sourcing for key intermediates may face margin compression or be forced to renegotiate contracts, which can ripple into tender behavior and availability across hospital channels.
Importantly, tariffs also influence strategic decision-making beyond direct cost. They can accelerate initiatives to strengthen domestic or allied-nation manufacturing footprints, encourage long-term supply agreements to stabilize economics, and push greater transparency in supplier-of-supplier relationships. For ceftazidime dihydrochloride, where trust in consistency and documentation is central, the tariff-driven need for supply-chain reconfiguration makes supplier qualification discipline and scenario planning indispensable rather than optional.
Segmentation signals show demand shaped by formulation practicality, dosing standardization, channel-specific procurement pressure, and differentiated care settings
Segmentation patterns for ceftazidime dihydrochloride reveal how buyers balance clinical fit, operational convenience, and procurement constraints. Across product form, powder for injection continues to command attention in settings that prioritize shelf stability and flexible preparation workflows, while ready-to-use or premixed presentations are evaluated for their ability to reduce compounding burden and medication-preparation errors in high-throughput acute-care environments. This form-driven distinction often maps directly to institutional capabilities, with larger hospitals and integrated delivery networks more willing to pay for workflow efficiency when staffing pressure and safety metrics dominate.When viewed through the lens of strength and dosing configuration, procurement preferences often align with standardized protocols for severe infections and renal-adjusted dosing pathways. Facilities seeking to minimize waste may prefer configurations that match common dose bands, while centers managing complex, critically ill populations tend to value the ability to titrate dosing without excessive vial splitting. In practice, the “best” strength is less about nominal volume and more about harmonizing pharmacy operations with evidence-based order sets.
Distribution channel segmentation underscores a widening gap between centralized purchasing and decentralized urgent need. Hospital pharmacies and group purchasing structures emphasize contract reliability, service-level guarantees, and recall responsiveness, whereas retail or community channels, where applicable, focus more on availability and substitution rules. This divergence affects how suppliers design their customer support, from tender documentation and electronic data interchange to short-dated inventory management.
End-use segmentation further differentiates demand by care intensity. Hospitals, particularly intensive care units and emergency departments, remain pivotal because they manage the highest-risk infections and the most complex empiric-to-targeted therapy transitions. Meanwhile, ambulatory infusion and post-acute settings increasingly influence continuity of therapy decisions, especially where early discharge protocols require stable access to injectable antibiotics. Taken together, these segmentation dynamics highlight a market where operational fit and supply assurance are as decisive as clinical equivalence, and where suppliers that tailor offerings to workflow realities can outperform those competing on price alone.
Regional contrasts reveal how contracting structures, regulatory intensity, and manufacturing concentration shape access and competitiveness across global markets
Regional dynamics for ceftazidime dihydrochloride reflect a mix of healthcare infrastructure maturity, regulatory stringency, and supply-chain architecture. In the Americas, purchasing decisions are heavily influenced by institutional contracting, shortage awareness, and heightened expectations for quality transparency. Buyers often scrutinize backup supply options and documentation readiness, and procurement teams increasingly partner with clinical leadership to align access with stewardship and formulary objectives.Across Europe, the Middle East, and Africa, variability is more pronounced. In Western Europe, centralized tenders and rigorous regulatory oversight can intensify price competition while simultaneously elevating compliance expectations. In parts of the Middle East, rapid healthcare capacity expansion and reliance on imports can heighten sensitivity to lead times and distributor performance. Several African markets face structural constraints related to cold-chain consistency, hospital funding cycles, and access to quality-assured injectables, making dependable distribution networks and training support particularly important.
Asia-Pacific stands out for its dual role as both a consumption region and a critical supply base for pharmaceutical manufacturing. Large, diverse patient populations and expanding hospital networks can sustain significant clinical need, while policy initiatives in several countries support domestic production capabilities. At the same time, the region’s prominence in upstream supply means that regulatory actions, environmental compliance measures, or logistics disruptions can have outsized downstream effects on global availability.
These regional contrasts matter because they shape what “winning” looks like. In some geographies, success is defined by tender strategy and regulatory excellence; in others, it hinges on distribution reliability, local partnerships, and the ability to manage import complexity. Companies that adapt their commercial and quality models to each region’s procurement logic and infrastructure realities are better positioned to maintain continuity and credibility over time.
Competitive advantage is defined by quality maturity, dependable sterile manufacturing, portfolio-level service, and partnerships that protect continuity of supply
Company performance in ceftazidime dihydrochloride is increasingly differentiated by execution rather than novelty. Leaders tend to demonstrate disciplined quality systems, consistent batch release performance, and mature supplier management that extends beyond first-tier vendors. This matters because hospital buyers and regulators alike interpret repeated quality events, documentation gaps, or volatile lead times as indicators of structural risk.A second axis of competition centers on portfolio and service breadth. Organizations that can support multiple sterile injectables, provide responsive pharmacovigilance and medical information, and offer dependable order fulfillment often gain preferred status in hospital procurement. Even when products are therapeutically interchangeable, customer experience in contracting, backorder communication, and recall handling can influence long-term relationships.
Manufacturing footprint strategy also separates strong players from fragile ones. Companies with geographically diversified capacity, validated technology transfer playbooks, and the ability to qualify alternate sources for key materials can navigate trade friction and logistics disruption with less customer impact. Meanwhile, firms dependent on single-site production or narrowly concentrated upstream sourcing may be forced into reactive changes that increase compliance workload and raise the probability of service interruptions.
Finally, collaboration capabilities are emerging as an advantage. Partnerships with contract manufacturing organizations, regional distributors, and local registration specialists can accelerate market access and improve service levels, provided governance is tight and quality oversight is robust. In a category where reliability is part of the clinical promise, companies that treat operational excellence as a commercial differentiator are positioned to sustain trust with both healthcare providers and procurement stakeholders.
Leaders can win by engineering resilience, aligning presentations to pharmacy workflow, modernizing quality systems, and contracting around service reliability
Industry leaders can strengthen their position in ceftazidime dihydrochloride by treating resilience as a design requirement. The first priority is to harden supply through qualified redundancy for critical inputs and at least one viable alternate manufacturing path, supported by pre-approved change-control scenarios. This approach reduces the time needed to respond to tariff-driven re-sourcing or unexpected site disruptions while preserving compliance integrity.Next, organizations should align product strategy with real pharmacy workflows. Investing in the right mix of presentations and strengths can reduce waste, support standardized dosing protocols, and improve user confidence in high-acuity settings. In parallel, strong medical affairs engagement with stewardship stakeholders can help ensure that positioning emphasizes appropriate use, compatibility with local guidelines, and clarity around preparation and administration.
Commercial execution should also evolve to match procurement realities. Contracting teams benefit from risk-sharing structures that emphasize service levels, transparency in allocation rules during constrained supply, and rapid communication pathways for hospital pharmacies. Where tenders dominate, disciplined bid governance and scenario-based pricing that accounts for trade and logistics volatility can protect relationships and reduce the likelihood of abrupt contract failures.
Finally, leaders should modernize quality and data practices to keep pace with regulatory expectations. Digital batch records, stronger analytics for deviation trending, and supplier-audit programs that extend into second-tier risk can improve inspection readiness and reduce quality-driven interruptions. When combined, these actions translate into a measurable advantage: fewer disruptions, stronger customer trust, and more predictable operational performance in an environment that increasingly rewards reliability.
A triangulated methodology combining expert interviews, regulatory and trade documentation review, and consistency checks builds a decision-ready evidence base
The research methodology for this analysis integrates structured primary engagement with rigorous secondary validation to ensure relevance for decision-makers in manufacturing, procurement, and commercialization. Primary inputs include interviews and discussions with stakeholders across the value chain, such as hospital pharmacy and procurement professionals, supply-chain and quality leaders, distributors, and subject-matter experts familiar with sterile and injectable antibiotic categories. These perspectives are used to test assumptions about purchasing criteria, switching frictions, and the operational impact of regulatory and trade developments.Secondary research consolidates publicly available information from regulatory databases, pharmacopoeial and standards documentation, government trade publications, tender portals where accessible, corporate disclosures, and peer-reviewed clinical and manufacturing literature relevant to cephalosporins and injectable antibiotics. This step establishes a consistent baseline for regulatory context, product positioning, and supply-chain considerations while avoiding reliance on non-permitted sources.
Data triangulation is applied throughout the process. Claims or themes identified in interviews are cross-checked against documentary evidence and reconciled through follow-up validation where inconsistencies appear. The analysis also uses framework-based evaluation to compare competitors on dimensions such as manufacturing footprint logic, quality posture, channel strategy, and customer service capability, focusing on decision-relevant differentiation rather than numerical projections.
Finally, the report applies editorial and technical review to maintain clarity, factual accuracy, and internal consistency. This includes harmonizing terminology for product forms and channels, verifying regulatory references, and ensuring that conclusions are anchored in observable market behavior and credible stakeholder input. The result is a practical, decision-oriented view of ceftazidime dihydrochloride that supports strategic planning, supplier evaluation, and risk management.
Ceftazidime dihydrochloride success now depends on resilient operations, tariff-aware sourcing, and tailored regional and channel strategies
Ceftazidime dihydrochloride remains an essential antibiotic in serious infection management, but the business environment around it has become more demanding. Stewardship-driven utilization patterns, higher expectations for supply assurance, and tighter quality scrutiny are shifting competitive advantage toward organizations that can execute reliably across manufacturing, compliance, and customer service.The tariff landscape in 2025 reinforces this reality by increasing the value of optionality and disciplined change management. Companies that understand their upstream exposure, maintain qualified alternatives, and communicate transparently with buyers are better positioned to sustain continuity and protect long-term relationships.
Segmentation and regional dynamics further emphasize that there is no single winning strategy. Product form and dosing configuration must fit pharmacy workflows, channels require tailored contracting and fulfillment capabilities, and regions differ widely in tender structures, infrastructure constraints, and regulatory intensity. In this context, leaders that operationalize resilience and align offerings with real-world care pathways can convert complexity into a durable advantage.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Ceftazidime Dihydrochloride Market
Companies Mentioned
The key companies profiled in this Ceftazidime Dihydrochloride market report include:- Anhui Huasheng Pharmaceutical Co., Ltd.
- Cangzhou Zhongcheng Pharmaceutical Co., Ltd.
- Hubei Biocause Pharmaceutical Co., Ltd.
- Hubei Yitai Pharmaceutical Co., Ltd.
- Jiangsu Hengrui Medicine Co., Ltd.
- Northeast Pharmaceutical Group Co., Ltd.
- Salvavidas Pharmaceutical Pvt. Ltd.
- Sandoz International GmbH
- Shandong Lukang Pharmaceutical Co., Ltd.
- Teva Pharmaceutical Industries Ltd.
- Zhejiang Huahai Pharmaceutical Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
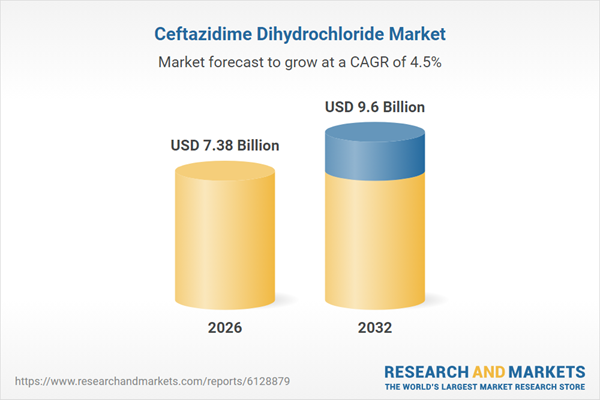
| Estimated Market Value ( USD | $ 7.38 Billion |
| Forecasted Market Value ( USD | $ 9.6 Billion |
| Compound Annual Growth Rate | 4.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |