Speak directly to the analyst to clarify any post sales queries you may have.
Cephalosporin C acylase as a strategic biocatalyst: why process performance, compliance, and supply resilience now converge
Cephalosporin C acylase sits at the center of a critical conversion step in the cephalosporin value chain, enabling the transformation of cephalosporin C into key intermediates used to manufacture widely deployed beta-lactam antibiotics. As the industry recalibrates around resilient supply, sustainability expectations, and stringent quality oversight, this enzyme has become more than a process reagent; it is increasingly a lever for operational performance, regulatory readiness, and long-term competitiveness.What elevates the strategic relevance of cephalosporin C acylase is the way it intersects with multiple decision domains at once. Technical leaders focus on activity, selectivity, stability, immobilization compatibility, and impurity profiles, while commercial teams weigh supplier reliability, lead times, and risk diversification. Meanwhile, executives track broader changes such as evolving trade policy, heightened scrutiny of antimicrobial manufacturing footprints, and the need to modernize facilities without disrupting validated processes.
In this context, an executive summary must do more than recount product fundamentals. It must translate technical and operational realities into decision-ready themes: where value is being captured in the chain, which risks are becoming non-negotiable, and how procurement and manufacturing strategies can be aligned with shifting policy and compliance demands. The discussion that follows frames those themes in a way that supports both near-term execution and longer-horizon strategy.
From activity metrics to end-to-end manufacturability: the shifts redefining enzyme selection, supplier qualification, and process design
The landscape for cephalosporin C acylase is undergoing a set of shifts that are less about a single breakthrough and more about a compounded redefinition of “best practice.” One major change is the rising premium placed on reproducibility across sites and batches. Manufacturers are increasingly unwilling to treat enzyme variability as an acceptable nuisance; instead, they expect tighter certificates of analysis, clearer provenance of production strains, and more transparent controls around fermentation, downstream purification, and formulation. This is especially pronounced where the enzyme step is a known driver of impurity formation or yield sensitivity.At the same time, process intensification is reshaping how buyers evaluate enzyme performance. The bar is moving from simple activity metrics toward performance under realistic plant constraints-temperature windows, solvent tolerance, substrate concentrations, and cycle time. As plants pursue higher throughput or lower energy use, enzymes that maintain selectivity under stress conditions, or that enable fewer downstream purification burdens, become strategically preferred even if unit pricing appears higher.
Another transformative shift is the growing role of immobilization, reuse, and continuous or semi-continuous operation concepts. Even when full continuous manufacturing is not adopted, many producers are integrating more advanced reactor configurations and resin-supported systems to stabilize biocatalysts and increase batch-to-batch consistency. This puts pressure on suppliers to offer enzyme formats engineered for predictable binding, minimal leaching, and stable kinetics over multiple cycles.
Sustainability and environmental compliance are also becoming embedded selection criteria rather than marketing narratives. Waste reduction, reduced use of harsh reagents, and improved E-factor considerations are increasingly tied to permitting and reputational expectations. Enzymatic routes that cut salt loads or reduce solvent intensity can strengthen a site’s license to operate. Consequently, decision-makers now evaluate cephalosporin C acylase not only as a yield driver but also as a tool to improve overall environmental performance.
Finally, the buyer-supplier relationship model is changing. Instead of transactional purchasing, more organizations are pursuing development-like collaboration with enzyme providers, particularly for strain improvement, tailored formulation, and on-site troubleshooting. This is driven by the need to derisk validation, secure continuity of supply, and shorten technology transfer timelines. The net effect is a market where technical support capability and documentation discipline increasingly differentiate suppliers, and where procurement strategies are expected to align tightly with quality and regulatory functions.
Trade policy as an operational variable: how anticipated 2025 U.S. tariffs reshape qualification rigor, landed cost stability, and sourcing risk
United States tariffs anticipated for 2025 introduce a complex layer of cost and compliance considerations for organizations connected to the cephalosporin intermediate supply chain. Even when cephalosporin C acylase itself is not directly targeted, tariffs on adjacent inputs-specialty chemicals, chromatography resins, fermentation nutrients, filtration hardware, single-use components, and cold-chain logistics-can alter landed costs and procurement behavior. The practical consequence is that enzymatic steps, once optimized largely for yield and quality, are now also evaluated through a lens of trade-exposed cost volatility.One immediate impact is a renewed emphasis on origin transparency and harmonized customs documentation. Importers are likely to require clearer declarations on manufacturing location, intermediate processing steps, and content classification to avoid clearance delays and disputes. For regulated pharmaceutical supply chains, any delay that forces production rescheduling can cascade into validation and release timing challenges. As a result, companies are tightening supplier onboarding and periodic review processes, pushing for stronger documentation packages and greater visibility into sub-supplier networks.
Tariff-driven pressure can also accelerate dual-sourcing and regionalization strategies. If cost differentials widen between regions, buyers may choose to qualify at least one alternative supplier in a different trade corridor, even at the expense of short-term complexity. However, qualifying a second enzyme source is not trivial; it can trigger comparability protocols, revalidation activities, and stability considerations. Therefore, organizations are increasingly weighing the total risk-adjusted cost of dual sourcing against the potential exposure of relying on a single import pathway.
Over the medium term, tariffs may influence where enzyme production capacity is expanded or upgraded. Suppliers with flexible manufacturing footprints, or with the ability to perform final formulation and packaging closer to U.S. demand centers, can reduce exposure to trade friction and improve responsiveness. Buyers, in turn, may prefer vendors capable of localized finishing, safety stock programs, and contractually defined continuity measures.
The cumulative effect is not simply price inflation; it is the elevation of trade policy into a strategic input for technical operations. Enzyme selection decisions that once centered on kinetics and stability now increasingly incorporate risk questions: How exposed is the supply route? How quickly can alternatives be validated? What contractual provisions ensure notification of process changes or site transfers? These considerations are likely to shape purchasing behavior throughout 2025, especially for organizations seeking to avoid disruptions in antibiotic intermediate production.
Segmentation signals that format choice, technology maturity, and end-use priorities determine who wins on consistency, support depth, and scalability
Segmentation reveals a market defined by the interplay of enzyme format, production method, end-use positioning, and procurement expectations. Across product forms, decision-makers often differentiate between formats optimized for straightforward integration into existing batch reactors and formats designed for longer operational life under reuse or immobilization regimes. This distinction influences not only total consumption but also the kind of technical service demanded, since longer-life formats typically require tighter control over handling, storage, and reactor compatibility.When viewed through production and technology lenses, buyers increasingly distinguish offerings based on the maturity of upstream fermentation control, downstream purification discipline, and the ability to maintain lot-to-lot consistency. Where production platforms incorporate more advanced strain engineering and process analytics, customers often experience improved predictability in activity and impurity profiles. In turn, these attributes can reduce downstream burden for intermediate purification and help stabilize finished API quality attributes.
Application-driven segmentation highlights the different performance and documentation thresholds between organizations using cephalosporin C acylase for high-volume intermediate manufacturing versus those using it for development, tech transfer, or specialized small-scale production. In high-throughput settings, productivity, robustness, and supply continuity dominate, while development-oriented users prioritize flexibility, technical collaboration, and rapid iteration on reaction parameters. These distinct needs shape how suppliers position technical support, packaging sizes, and change-control communication.
End-user segmentation also underscores varied buying criteria across integrated manufacturers, contract manufacturers, and research-driven organizations. Integrated producers tend to emphasize strategic supply security, auditing rights, and long-term agreements that stabilize operations. Contract manufacturers often seek supplier responsiveness, documented consistency, and the ability to support multi-client compliance requirements. Research-centric users may accept smaller lots but demand detailed characterization and support for method development.
Finally, procurement segmentation shows an emerging split between buyers who prefer standardized catalog-style offerings and those who increasingly require customized formulations, immobilization-ready preparations, or tailored specifications that match their validated process windows. This trend reflects a broader shift toward fit-for-purpose enzymes, where the “best” product is defined less by generic activity and more by the reliability of performance in the customer’s exact operating context.
Regional realities show how regulation intensity, biotech capacity, and supply-chain infrastructure shape adoption patterns and vendor fit
Regional dynamics for cephalosporin C acylase are strongly shaped by antibiotic manufacturing footprints, regulatory enforcement patterns, and the maturity of industrial biotechnology ecosystems. In the Americas, supply-chain resilience and documentation rigor are central themes, particularly where buyers anticipate trade friction and prioritize continuity plans. This environment tends to reward suppliers that can provide robust audit readiness, clear change-control commitments, and flexible logistics options.Across Europe, the market is heavily influenced by stringent quality expectations and increasingly prominent sustainability considerations. Decision-makers often weigh not only process performance but also environmental compliance posture, waste treatment implications, and the ability to demonstrate responsible manufacturing practices. This encourages adoption of enzyme solutions that help reduce harsh reagents or improve overall process efficiency, especially where environmental permitting and community expectations are high.
In the Middle East and Africa, the landscape is heterogeneous, with demand shaped by evolving local manufacturing ambitions, procurement modernization, and varying levels of domestic capability. Buyers in this region often balance the need for reliable imported materials with the desire to build local or regional capacity over time. Suppliers that can support training, technology transfer, and reliable cold-chain or controlled logistics tend to be better positioned.
Asia-Pacific remains central to both production and consumption, with strong capabilities in fermentation-based manufacturing and broad antibiotic supply chain participation. Competitive intensity is high, and customers frequently expect rapid delivery, technical responsiveness, and cost-effective performance without compromising on consistency. As regulatory expectations continue to tighten in multiple jurisdictions, suppliers that pair scale advantages with strong documentation and quality systems can stand out.
Across all regions, a common thread is the convergence of regulatory readiness and operational efficiency. Regional strategies increasingly depend on how well suppliers can support audits, provide traceability, and maintain stable performance despite the logistical realities of cross-border shipping, storage constraints, and site-to-site process variation.
Competitive advantage is shifting toward documentation discipline, continuity assurances, and application-led support - not just enzyme performance claims
Company competition in cephalosporin C acylase is increasingly defined by a combination of biochemical capability and industrial discipline. Leading participants tend to differentiate through consistent enzyme performance, strong quality management systems, and the ability to provide documentation packages aligned with regulated pharmaceutical manufacturing. For many buyers, the supplier’s credibility is built as much on change-control maturity and deviation handling as it is on headline activity values.A second axis of differentiation is technical partnership capacity. Companies that can support reaction optimization, immobilization strategy, troubleshooting, and scale-up guidance are often preferred for long-term relationships. This is particularly relevant when a buyer is seeking to intensify production, reduce impurity formation, or shorten cycle times. In these cases, the supplier’s application scientists and process engineers become a practical extension of the customer’s own technical team.
Manufacturing footprint and business continuity planning also matter more than before. Suppliers that can demonstrate redundancy in critical steps, controlled raw material sourcing, and validated cold-chain handling can reduce the perceived risk for buyers. As trade and logistics uncertainty increases, vendors that provide clear lead-time commitments, safety stock programs, and transparent communication during disruptions are more likely to retain strategic accounts.
Finally, innovation in enzyme engineering and formulation continues to influence competitive positioning, especially where customers demand improved stability, higher tolerance to process conditions, or compatibility with specific reactor and purification setups. Companies that invest in iterative improvement-while maintaining strict comparability controls-can help customers modernize processes without triggering unnecessary regulatory complexity. In a market where switching costs can be high, trust, documentation discipline, and sustained technical performance form the foundation of durable competitive advantage.
Industry leaders can win by linking enzyme choices to risk registers, tariff-aware sourcing, and incremental intensification with stronger governance
Industry leaders can strengthen their position by treating cephalosporin C acylase as a strategic component of operational risk management rather than a routine consumable. The first recommendation is to formalize enzyme criticality assessments that connect performance variability to downstream quality outcomes. By quantifying how enzyme attributes influence impurity formation, yield sensitivity, and rework rates, organizations can justify targeted investments in higher-consistency grades or deeper supplier partnerships.Next, companies should build tariff-aware sourcing playbooks that include origin mapping, customs classification checks, and scenario-based landed cost reviews for enzyme-adjacent inputs. This work is most effective when procurement, quality, and supply chain teams share a single risk register and coordinate supplier communications. Where dual sourcing is necessary, leaders should design comparability and validation pathways early, ensuring that alternate suppliers are qualified before disruption forces rushed decisions.
Process teams can also pursue practical intensification without overcommitting to disruptive manufacturing redesign. Priorities often include improved control of reaction conditions, evaluation of immobilization or reuse options where feasible, and tighter monitoring of enzyme activity decay across runs. These measures can reduce batch variability while enabling incremental productivity gains within existing facility constraints.
Finally, leaders should elevate supplier governance. Stronger technical agreements, clearer change-notification requirements, periodic data reviews, and joint problem-solving routines can prevent surprises that otherwise appear as deviations on the plant floor. When paired with collaborative development support, governance becomes a lever for both compliance resilience and continuous improvement-two outcomes that will matter more as regulatory scrutiny and trade uncertainty remain elevated.
A rigorous mixed-method approach combining technical literature, policy review, and expert validation to ground decisions in operational reality
The research methodology integrates structured secondary review with primary engagement to ensure that conclusions reflect both documented industry direction and operational reality. The work begins by compiling and triangulating information from regulatory publications, pharmacopoeial and quality guideline frameworks, patent and scientific literature, trade and customs policy updates, and public company disclosures relevant to enzyme manufacture and beta-lactam intermediate processing.Primary research is then used to validate assumptions and sharpen practical interpretation. Interviews and consultations are conducted with stakeholders spanning enzyme suppliers, antibiotic intermediate manufacturers, contract manufacturing organizations, quality and regulatory professionals, and process development experts. These discussions focus on procurement criteria, qualification practices, documentation expectations, performance-in-use considerations, and the operational implications of logistics and policy shifts.
Insights are consolidated using a consistent analytical framework that emphasizes drivers, constraints, and decision points rather than speculative projections. Where conflicting perspectives appear, the approach prioritizes reconciliation through additional validation and cross-referencing, ensuring that the final narrative reflects consensus patterns as well as clearly bounded differences in practice across end users.
Finally, findings undergo internal consistency checks to confirm that segmentation logic, regional narratives, and competitive insights align with the technical realities of cephalosporin C acylase deployment. This ensures the executive summary supports practical decision-making for strategy, procurement, and technical leadership teams.
The path forward centers on predictable performance and resilient sourcing, turning a critical enzyme step into a durable operational advantage
Cephalosporin C acylase remains a technically specialized enzyme, but its business significance is expanding as manufacturers confront heightened expectations for reliability, compliance, and resilient sourcing. The market’s direction is being shaped by a shift toward end-to-end manufacturability, where enzyme selection reflects not only catalytic performance but also documentation quality, supplier governance, and compatibility with intensified operations.As anticipated U.S. tariff changes in 2025 ripple through adjacent inputs and logistics, trade exposure becomes a tangible variable in procurement and qualification strategies. Companies that proactively map origin risks, strengthen documentation discipline, and build qualified alternatives will be better prepared to protect production continuity.
Across segments and regions, the consistent message is that competitive advantage increasingly comes from operational predictability. Organizations that integrate technical, quality, and supply chain decision-making-while partnering with capable suppliers-will be positioned to improve process robustness and navigate uncertainty without sacrificing compliance or performance.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
19. China Cephalosporin C Acylase Market
Companies Mentioned
The key companies profiled in this Cephalosporin C Acylase market report include:- Amano Enzyme Inc.
- Codexis, Inc.
- DuPont de Nemours, Inc.
- Enzymicals AG
- Evonik Industries AG
- Koninklijke DSM N.V.
- Lonza Group AG
- Novozymes A/S
- Seikagaku Corporation
- Shionogi & Co.
- Wacker Chemie AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | January 2026 |
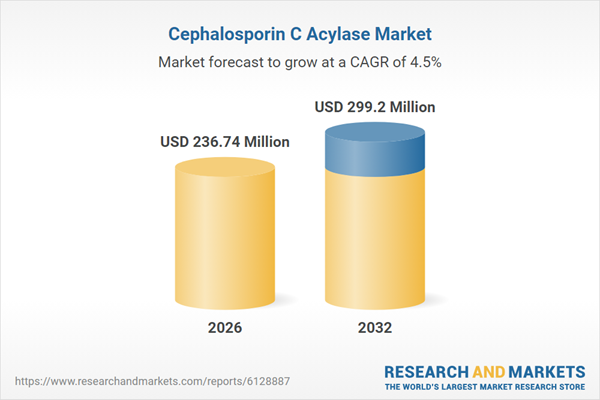
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 236.74 Million |
| Forecasted Market Value ( USD | $ 299.2 Million |
| Compound Annual Growth Rate | 4.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |