Speak directly to the analyst to clarify any post sales queries you may have.
Why cleanroom shoes have become a frontline contamination-control technology shaping compliance outcomes, worker safety, and throughput stability
Cleanroom shoes sit at the intersection of contamination control, operator safety, and operational continuity. In controlled environments-whether supporting semiconductor yields, sterile drug production, medical device assembly, or precision optics-footwear is not a minor accessory but a continuous interface between people, surfaces, and airflow patterns. As a result, procurement teams, EHS leaders, and quality units increasingly treat cleanroom footwear as an engineered control that must meet defined performance requirements for particle shedding, electrostatic discharge, chemical resistance, and ergonomic stability.In parallel, the market is being shaped by a more demanding compliance climate and heightened sensitivity to supply risk. Buyers are aligning footwear specifications with cleanroom classifications, gowning protocols, and facility-specific SOPs, while also insisting on documentation that supports audits and customer requirements. The conversation has shifted from “what is available” to “what can be validated,” emphasizing consistent lot-to-lot quality, traceable materials, and predictable delivery.
At the same time, innovation is accelerating in materials, outsole design, and process cleanliness. Manufacturers are refining low-shedding polymers, improving slip resistance without increasing particle generation, and designing options that reduce fatigue during long shifts. As end users pursue higher throughput and fewer deviations, cleanroom shoes are increasingly viewed as a lever to reduce excursions, streamline gowning, and maintain stable production under tighter regulatory scrutiny.
How materials science, lifecycle economics, and compliance-first procurement are reshaping cleanroom footwear expectations and supplier competition
The competitive landscape for cleanroom shoes is undergoing transformative shifts driven by technology convergence and stronger governance expectations. Cleanroom operators are tightening the link between footwear performance and total contamination-control strategy, integrating shoe selection into broader programs such as ESD management, cleanroom housekeeping chemistry, and gowning room ergonomics. This integration raises the bar for suppliers, who are now expected to provide not only products but also validation support, change-control transparency, and guidance for use conditions that can affect shedding and durability.Materials engineering is one of the most visible shifts. Demand is rising for polymers and composite constructions that balance low particle generation with durability and chemical tolerance. Outsole formulations are being optimized to minimize abrasion on cleanroom floors while maintaining traction in environments where disinfectants, solvents, or process residues can change surface friction. Meanwhile, clean construction methods and packaging practices are becoming more central differentiators, with buyers scrutinizing how shoes are cleaned, bagged, and transported to preserve cleanliness levels.
Another shift is the move toward lifecycle thinking. Rather than evaluating shoes purely on unit price, facilities are considering wear life, cleaning compatibility, replacement cadence, and the operational cost of nonconformance events. This perspective favors suppliers that can demonstrate performance consistency across lots and offer guidance on inspection, storage, and rotation policies. Alongside this, sustainability expectations are emerging, especially where organizations pursue waste reduction through longer-lasting designs, reusable models where appropriate, and packaging optimization-without compromising cleanroom requirements.
Finally, the landscape is being reshaped by global supply-chain realignment. Manufacturers and distributors are diversifying sourcing, improving regional stocking strategies, and reassessing where cleaning, finishing, and packaging occur. This is not simply a logistics adjustment; it affects lead times, documentation practices, and the ability to respond to demand spikes in regulated industries. As a result, supplier qualification increasingly considers resilience, traceability, and responsiveness as core performance attributes rather than secondary purchasing criteria.
What the 2025 U.S. tariff environment means for cleanroom footwear sourcing, qualification timelines, and resilience planning across global supply chains
The 2025 United States tariff environment introduces a new layer of complexity for cleanroom shoe supply chains that already depend on tightly controlled materials and specialized manufacturing steps. Footwear components-such as polymer uppers, specialty outsole compounds, ESD-related additives, and packaging materials-can span multiple countries of origin before final assembly or clean packaging. When tariffs affect any link in that chain, the impact is rarely limited to higher landed cost; it can trigger requalification needs, documentation updates, and timeline disruptions that ripple into production schedules.One immediate consequence is a renewed emphasis on country-of-origin transparency and tariff engineering within compliant boundaries. Importers and distributors are more likely to refine classification practices, verify bills of materials, and seek clearer supplier attestations to reduce exposure to unexpected duties. However, cleanroom footwear cannot be freely substituted like commodity items; material changes can alter shedding behavior, chemical resistance, or ESD performance. Therefore, tariff-driven sourcing shifts may require validation testing and quality approvals that extend beyond typical procurement cycles.
Tariffs also influence inventory strategy. To avoid interruptions, many buyers are increasing safety stocks for critical SKUs, especially those tied to qualification and audit readiness. Yet inventory expansion in cleanroom consumables and apparel has practical limits, including storage conditions, packaging integrity, and the risk of specification drift if product revisions occur. Consequently, buyers are balancing buffer inventory with supplier agreements that prioritize allocation and stable lead times.
Over the medium term, tariffs can accelerate nearshoring and multi-regional manufacturing footprints, but these transitions are constrained by clean manufacturing capacity and the specialized know-how required for controlled-environment products. As companies reconfigure supply, they may shift more value-added steps-such as clean finishing, laundering-equivalent processes, or final bagging-closer to U.S. distribution hubs. This can reduce tariff exposure on certain inputs while improving responsiveness, but it also places a premium on process controls and auditability at newly utilized sites.
For end users, the practical takeaway is that tariff impacts will be felt as a blend of cost pressure, qualification workload, and risk management demands. Organizations that proactively map their footwear supply chain, lock specifications with clear change-notification terms, and build dual-sourcing pathways where feasible will be better positioned to maintain compliance and operational stability under a more volatile trade regime.
Segmentation insights show cleanroom footwear choices hinge on validated use cases, from material behavior and reuse models to end-use compliance demands
Segmentation reveals how cleanroom shoe requirements are shaped less by generic product categories and more by specific contamination-control and safety outcomes. When viewed through product type lenses such as reusable and disposable cleanroom shoes, the operational logic becomes clear: reusable models are favored where facilities have established protocols for controlled reuse, inspection, and rotation, while disposable options are often selected to simplify changeover discipline in high-sensitivity zones or where cross-area movement raises contamination concerns. This distinction becomes especially consequential when audit expectations demand that the chosen approach be supported by SOPs, training, and documented control points.Material segmentation highlights a second layer of decision-making. Polyurethane, PVC, and other engineered polymers each bring different shedding profiles, flexibility, and chemical resistance characteristics. Facilities using aggressive disinfectant regimes often prioritize materials that maintain integrity under repeated exposure, while ESD-managed environments weigh how material choices interact with flooring systems and grounding pathways. In practice, material selection is also tied to comfort and fatigue reduction, as long shifts can drive noncompliant behavior if shoes are uncomfortable or difficult to don within gowning workflows.
End-use segmentation clarifies why a single “best” cleanroom shoe rarely exists. Semiconductor and electronics environments typically prioritize ESD performance alongside low particle generation, while pharmaceutical and biotechnology operations emphasize cleanliness assurance, compatibility with sanitization chemicals, and strong documentation aligned to regulated manufacturing. Medical device manufacturers often operate across mixed clean classifications and may require footwear that transitions effectively between zones without compromising traction or generating debris. Food and beverage clean zones, where applicable, place heavier weight on slip resistance and washdown compatibility, while research laboratories often favor flexibility to support diverse protocols and quicker procurement cycles.
Application segmentation-such as use across controlled manufacturing, packaging areas, and support spaces-further explains purchasing behavior. A facility may standardize one validated model for the most critical rooms while using a different, simpler option in adjacent controlled corridors or material staging areas. In those situations, the governance challenge is ensuring that zone-based rules are intuitive and enforceable, with clear visual cues and storage practices to prevent mix-ups.
Distribution channel segmentation also influences outcomes. Direct procurement from manufacturers can support deeper technical alignment and change-control visibility, while distributor-led purchasing can improve availability, consolidate spend, and simplify replenishment across multiple sites. Many organizations adopt hybrid strategies, reserving direct relationships for high-criticality SKUs and leveraging distribution partners for breadth, rapid fulfillment, and regional stocking. Across these segmentation angles, the central insight is that cleanroom footwear programs succeed when specifications are aligned to validated use cases, and when procurement structures reinforce-not undermine-those controls.
Regional insights reveal how compliance culture, manufacturing density, and supply resilience priorities shape cleanroom shoe requirements across major markets
Regional dynamics underscore that cleanroom footwear demand is driven by the local density of regulated manufacturing, the maturity of contamination-control culture, and the structure of supply and distribution networks. In the Americas, buyers commonly emphasize audit readiness, documentation completeness, and responsive replenishment that supports continuous production. The United States in particular shows heightened sensitivity to trade policy, lead-time reliability, and the ability to lock specifications under clear change-control procedures. Across North America, ESD considerations remain prominent in electronics-related clusters, while life sciences corridors sustain demand for chemically compatible, low-shedding options that integrate smoothly into gowning SOPs.In Europe, Middle East & Africa, a strong regulatory and quality framework often translates into rigorous supplier qualification and a preference for standardized programs deployed across multiple sites. European manufacturers frequently evaluate footwear in the context of broader cleanroom garment systems, seeking consistency in contamination performance and compatibility with local disinfectant practices. In addition, cross-border procurement within the region increases the value of suppliers that can provide harmonized documentation and stable product availability across different national requirements and languages.
In Asia-Pacific, the concentration of electronics, semiconductor, and precision manufacturing drives high expectations for ESD performance, clean packaging discipline, and reliable high-volume supply. The region’s manufacturing scale can amplify the operational impact of footwear decisions, making consistency and defect prevention central purchasing themes. At the same time, organizations operating multi-site networks across Asia-Pacific often pursue supplier strategies that balance local availability with global standardization, aiming to reduce variability while maintaining flexibility in logistics.
Across all regions, cleanroom footwear purchasing is increasingly influenced by the ability to support regional stocking, rapid replenishment, and predictable documentation. As manufacturing footprints globalize and quality expectations converge, regional differences are narrowing in terms of baseline cleanliness and safety requirements, while diverging more on supply resilience, lead-time expectations, and how buyers manage tariff exposure, shipping complexity, and localized compliance nuances.
Company insights highlight that winners pair controlled manufacturing and documentation rigor with ergonomics, ESD credibility, and resilient fulfillment models
Company strategies in the cleanroom shoe space increasingly differentiate on validation support, manufacturing discipline, and the ability to sustain consistent performance under real operating conditions. Leading suppliers typically invest in controlled manufacturing environments, robust QA systems, and packaging processes designed to preserve cleanliness through transport and storage. Beyond the physical product, they strengthen trust by offering technical documentation, lot traceability practices, and clear communication pathways for product changes that could affect qualification.Product portfolios are also evolving to address both contamination control and human factors. Manufacturers that pair low-shedding constructions with improved ergonomics-such as better fit systems, fatigue-reducing insoles, and stable outsoles-are responding to a critical reality: comfort influences compliance. In high-discipline environments, even minor friction points in donning and doffing can increase the risk of procedural shortcuts, so suppliers that design for usability can indirectly improve contamination-control adherence.
Another competitive axis is ESD performance credibility. Companies serving electronics and semiconductor customers tend to emphasize repeatable electrical characteristics, compatibility with facility grounding systems, and guidance on how wear, cleaning, and humidity can affect outcomes. Where suppliers provide clearer testing approaches and usage guidance, they reduce the burden on end users who must defend their choices during audits or customer inspections.
Finally, companies are adjusting commercial models to match buyer expectations for resilience. Regional warehousing, distributor partnerships, and multi-origin manufacturing options are increasingly important, especially when trade dynamics or shipping disruptions threaten continuity. Suppliers that can demonstrate stable lead times, responsive issue resolution, and disciplined change notification are better positioned to become standardized partners rather than intermittent vendors.
Actionable recommendations help leaders standardize cleanroom footwear, reduce audit risk, and build resilient sourcing without sacrificing operator compliance
Industry leaders can strengthen cleanroom footwear programs by treating shoe selection as a controlled specification rather than a rotating commodity purchase. Start by linking footwear requirements directly to room classifications, floor types, disinfectant chemistry, and ESD governance, then translate those needs into measurable acceptance criteria. When specifications are clear, supplier comparisons become more objective, and internal stakeholders-quality, EHS, operations, and procurement-can align on what “fit for use” truly means.Next, build qualification pathways that anticipate change. Establish clear requirements for supplier change notifications, including material substitutions, manufacturing site changes, and packaging modifications. Where feasible, validate more than one approved model for critical areas to reduce single-source dependence, but ensure that dual sourcing does not introduce confusing gowning rules or inconsistent performance. In parallel, implement receiving and in-use inspection practices that focus on packaging integrity, visual defects, and indicators of material degradation, especially in environments with frequent chemical exposure.
To manage tariff and logistics volatility, develop a risk-based inventory and contracting strategy. For the most critical SKUs, consider allocation agreements, defined lead-time commitments, and regional stocking expectations. At the same time, avoid overstocking without controls; define storage conditions, first-in-first-out practices, and periodic reviews to ensure stocked items still match the approved specification and documentation set.
Operationally, improve compliance by designing footwear programs around human behavior. Standardize sizing, donning aids, and storage layouts in gowning rooms to reduce errors and speed shift changes. Reinforce training with simple rationale-how footwear affects particle transport and ESD outcomes-so adherence is not merely procedural but understood. Finally, track performance through incident reviews, user feedback, and supplier corrective-action responsiveness, using those signals to refine specifications and vendor scorecards over time.
Methodology built on triangulated primary interviews and structured secondary analysis to translate cleanroom footwear complexity into decisions teams can execute
The research methodology integrates structured secondary research, targeted primary engagement, and rigorous synthesis to ensure practical relevance for decision-makers in contamination-controlled environments. The work begins with a comprehensive mapping of the cleanroom shoe ecosystem, including product constructions, material options, cleanliness and ESD considerations, packaging practices, and the ways footwear is specified and qualified in regulated operations.Secondary research consolidates publicly available information such as company product literature, technical documentation practices, regulatory and standards context applicable to cleanroom operations, and trade and logistics considerations impacting controlled-environment supplies. This step establishes a baseline understanding of how suppliers position offerings, how requirements differ by industry, and where operational constraints frequently emerge.
Primary research then deepens the analysis through interviews and structured discussions with industry participants across the value chain. Engagement focuses on procurement and quality expectations, qualification and change-control practices, real-world performance tradeoffs, and the operational friction points that influence compliance. Perspectives from manufacturers, distributors, and end users are triangulated to reduce bias and to capture how priorities vary across regulated industries and geographies.
Finally, findings are synthesized using a segmentation-driven framework to translate qualitative inputs into actionable insights. The analysis emphasizes consistency checks across sources, careful handling of terminology differences between industries, and validation of themes through iterative review. The result is a decision-oriented view of cleanroom footwear that highlights risk management, specification design, supplier strategy, and operational execution rather than relying on unsupported assumptions.
Conclusion ties together compliance, usability, and supply resilience as the defining pillars of modern cleanroom footwear programs under scrutiny
Cleanroom shoes are increasingly recognized as a controllable variable that influences contamination outcomes, ESD integrity, and workforce safety. As manufacturing environments become more exacting, footwear programs must keep pace with rising expectations for documentation, performance consistency, and change-control discipline. This elevates the role of procurement and quality collaboration, ensuring that what is purchased can be defended, validated, and sustained.The industry is simultaneously navigating material innovation, lifecycle cost scrutiny, and supply-chain volatility shaped by trade policy and regional logistics constraints. In this environment, the strongest programs are those that connect segmentation-driven needs to clear specifications, qualify suppliers with resilience in mind, and design gowning workflows that support user compliance.
By approaching cleanroom footwear as a system-products, people, processes, and suppliers-organizations can reduce operational surprises and strengthen audit readiness. The path forward favors disciplined standardization where it matters most, paired with flexible sourcing strategies that protect continuity without eroding contamination-control rigor.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Cleanroom Shoes Market
Companies Mentioned
The key companies profiled in this Cleanroom Shoes market report include:- 3M Company
- Aditya Enterprise
- Ansell Limited
- Aramark Services Inc
- Berkshire Corporation
- Biomed Laboratories
- Blue Thunder Technologies
- Cardinal Health
- Cleanroomshop.com LLC
- Contec Inc
- Dastex Reinraumzubehör GmbH & Co KG
- DuPont de Nemours and Company
- Estatec
- Gaible
- Genev
- High‑Tech Conversions
- Honeywell International Inc
- Illinois Tool Works Inc
- ITW Texwipe LLC
- Kimberly‑Clark Corporation
- Lakeland Industries Inc
- LEENOL Industrial Co Ltd
- Micronclean Limited
- Micronova Manufacturing Inc
- Nupon Technology Phils Corp
- Prudential Overall Supply
- Shanghai Hanyang Clean Technology
- Superior Uniform Group Inc
- Taica Corporation
- Tarun Gloves
- Terra Universal Inc
- Texas Technologies Inc
- Unique Safety Services
- Valutek Inc
- VWR International LLC
- Westec Environmental Solutions Ltd
- Zhejiang CONCO Antistatic Technology Co Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
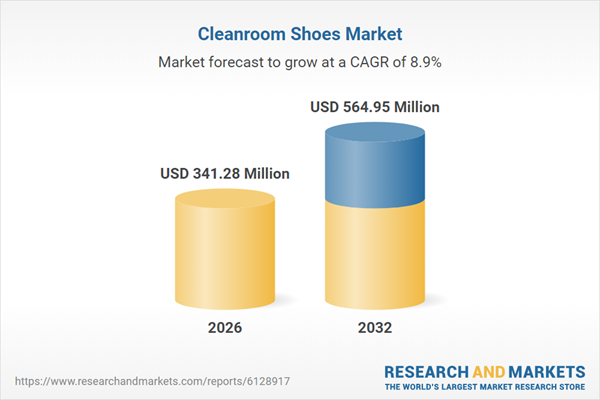
| Estimated Market Value ( USD | $ 341.28 Million |
| Forecasted Market Value ( USD | $ 564.95 Million |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 38 |