Speak directly to the analyst to clarify any post sales queries you may have.
AI-powered medical devices are redefining the landscape of healthcare innovation, offering new ways for providers to diagnose, monitor, and treat patients more efficiently and intelligently. As artificial intelligence continues to advance, senior decision-makers in healthcare and medtech are increasingly looking to capitalize on cutting-edge technologies that are transforming both clinical and operational outcomes.
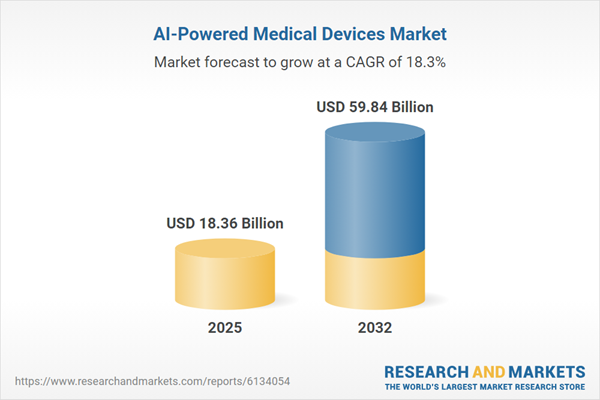
Market Snapshot: AI-Powered Medical Devices Market
The AI-powered medical devices market grew from USD 15.64 billion in 2024 to USD 18.36 billion in 2025. It is expected to continue growing at a CAGR of 18.26%, reaching USD 59.84 billion by 2032. This sustained expansion is driven by rapid technological integration, expanded adoption across clinical environments, and evolving reimbursement frameworks that support the implementation of intelligent healthcare solutions.
Scope & Segmentation of the AI-Powered Medical Devices Market
- Product Categories: Diagnostic equipment, imaging systems, infusion devices, monitoring equipment, surgical robots.
- Component Types: Hardware (processors, sensors, storage devices), services (consulting, maintenance, training), and software (application software, operating systems).
- Deployment Modes: Cloud platforms (hybrid, public, private) and on-premise installations.
- Technologies: Computer vision, deep learning, machine learning, natural language processing, robotics and automation.
- Application Areas: Cardiology (ECG analysis, echocardiography, hemodynamic monitoring), neurology (EEG analysis, neuroimaging), oncology (cancer screening, tumor analysis), orthopedics, and radiology.
- End Users: Ambulatory centers, diagnostic centers, home care, hospitals, research institutes.
- Sales Channels: Direct sales and distributors.
- Regions Covered: Americas (North America, Latin America), Europe, Middle East & Africa, Asia-Pacific.
Key Takeaways for Senior Decision-Makers
- AI has become integral to all stages of the device lifecycle—from design and diagnostics to research and post-market surveillance—streamlining medical workflows and improving operational efficiency.
- Technological advances such as deep learning, computer vision, and real-time predictive analytics are enabling smarter and more adaptive devices that tailor interventions for individualized patient care.
- Integration of AI with robotic surgical systems and infusion pumps is elevating procedural precision and reducing complication rates, while connected monitoring equipment allows for continuous patient data collection and adaptive responses.
- Modular system architectures and open interfaces support interoperability, speeding innovation cycles and simplifying integration with existing health data infrastructures.
- Robust cybersecurity and compliance with evolving regulations are critical, underpinning trust among care providers and ensuring patient data safety as AI becomes more deeply embedded in clinical practice.
- Strategic partnerships—particularly between medtech manufacturers and software providers—are driving rapid market entry and the successful scaling of AI-driven solutions.
Tariff Impact and Global Supply Chain Dynamics
New US tariff structures in 2025 have altered global supply chains for AI-enabled medical devices. Elevated import duties on components have prompted manufacturers to diversify sourcing, shift assembly to domestic or allied free trade zones, and prioritize the use of standardized subcomponents from local suppliers. This shift supports more agile R&D investment, encourages nearshoring practices, and accelerates regulatory approvals for domestically assembled products while maintaining safety and performance standards.
Methodology & Data Sources
This report is built on a mixed-methods research strategy, combining direct interviews with industry leaders and regulatory officials, in-depth analysis of academic literature and technical publications, and cross-validation with clinical and procurement records. Comprehensive segmentation and geographic analysis leverage both quantitative and qualitative stakeholder feedback, ensuring actionable market intelligence.
Why This Report Matters for Strategic Decision-Making
- Empower executives with clear visibility into competitive positioning, new partnership opportunities, and regulatory developments in the AI-powered medical devices market.
- Facilitate informed investment and vendor selection decisions by offering a robust segmentation framework and regional market analysis tailored to emerging trends and operational realities.
Conclusion
AI-powered medical devices are reshaping the healthcare value chain with smarter, more adaptive solutions. Forward-thinking leaders are leveraging these advances to optimize care pathways and sustain long-term innovation within a dynamic regulatory and technological environment.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this AI-Powered Medical Devices market report include:- GE HealthCare Technologies, Inc.
- Siemens Healthineers AG
- Koninklijke Philips N.V.
- Canon Medical Systems Corporation
- Fujifilm Holdings Corporation
- Samsung Electronics Co., Ltd.
- Medtronic plc
- Abbott Laboratories
- Johnson & Johnson Service, Inc.
- Zimmer Biomet Holdings, Inc
- NVIDIA Corporation
- Exo Imaging, Inc
- Empatica Inc.
- CLEW Medical Ltd.
- Eko Health, Inc.
- Medasense Biometrics Ltd.
- Oncora Medical
- Novo Nordisk A/S
- aetherAI Co., Ltd.
- Tandem Diabetes Care, Inc.
- Becton, Dickinson and Company
- Moon Surgical
- Vicarious Surgical Inc.
- Oracle Corporation
- Clairity, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | October 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 18.36 Billion |
| Forecasted Market Value ( USD | $ 59.84 Billion |
| Compound Annual Growth Rate | 18.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |