Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive Insight into the Dynamics of Osimertinib Generic Developments and Their Strategic Significance in Advancing Precision Oncology Treatment Paradigms
The landscape of oncology therapeutics has been fundamentally transformed by the introduction of targeted treatments, among which osimertinib has emerged as a leading third-generation epidermal growth factor receptor inhibitor. As patents on this pivotal therapy conclude, the emergence of generics presents both opportunities and challenges for manufacturers, healthcare providers, and patients alike. This report offers a detailed exploration of the factors shaping the entry of generic osimertinib products into global markets and the implications for clinical practice and commercial strategies.In recent years, the demand for cost-effective oncological treatments has surged, driven by the imperative to broaden patient access and contain healthcare expenditures. The transition from branded to generic formulations of osimertinib is poised to play a crucial role in achieving these objectives. Amidst evolving regulatory frameworks and heightened competitive intensity, stakeholders must navigate complex dynamics to capitalize on the growing acceptance of generic generics. This introduction establishes the foundations for understanding the multifaceted environment in which osimertinib generics will compete and thrive.
Illuminating the Transformative Technological and Regulatory Shifts Reshaping the Osimertinib Generics Landscape Across Global Pharmaceutical Ecosystems
In recent years, the osimertinib generics arena has been reshaped by technological innovations, progressive regulatory reforms, and shifting competitive landscapes. Advances in process chemistry and formulation development have enabled manufacturers to achieve enhanced bioequivalence with branded products while optimizing production yields. Concurrently, digital platforms for pharmacovigilance are providing real-time feedback on product performance, driving continuous improvements in quality and safety.Regulatory bodies across major markets have introduced streamlined pathways for generic approvals, reducing time to market without compromising rigorous standards. Such initiatives have spurred collaborations between contract development and manufacturing organizations and generic drug developers, fostering integrated supply chain models. Furthermore, patent expiries and settlements have unlocked opportunities for first-to-file exclusivity incentives, further accelerating the entry of generics into the competitive arena. Together, these transformative shifts underscore the dynamic ecosystem that industry participants must navigate to successfully commercialize osimertinib generics.
Analyzing the Comprehensive Implications of 2025 United States Tariff Adjustments on Osimertinib Generic Drug Supply Chains and Competitive Pricing Dynamics
The implementation of new tariff schedules by the United States in 2025 has introduced significant variables into the cost structures of generic osimertinib supply chains. Import duties on active pharmaceutical ingredients have elevated procurement expenses, prompting manufacturers to reassess sourcing strategies and negotiate revised terms with key suppliers. These adjustments have had downstream effects on contract manufacturing agreements and pricing models.Moreover, altered tariff classifications have incentivized certain producers to relocate or expand manufacturing operations within tariff-exempt jurisdictions, thereby stabilizing input costs and ensuring continuity of supply. Despite these adaptations, the increased duties have necessitated the adoption of hedging mechanisms and inventory optimization practices to mitigate volatility. As a result, stakeholders are now placing greater emphasis on resilient supply chain frameworks that can absorb tariff-induced disruptions while maintaining competitive pricing for downstream partners and end users.
Unveiling Critical Segment Performance Drivers Across Dosage Strengths and Distribution Channels Defining the Competitive Contours of Osimertinib Generics
An in-depth exploration of dosing and distribution segmentation reveals critical insights into where value creation and competitive advantage can be realized. Analysis of dose strengths considers the clinical preferences and prescribing patterns for 40 milligram and 80 milligram formulations, highlighting how each strength aligns with therapy lines and patient adherence profiles. This examination underscores the necessity for manufacturers to tailor production capacity and marketing initiatives to the nuanced demands of each dosage category.Parallel evaluation of distribution channels assesses the roles of traditional brick-and-mortar pharmacies alongside digital platforms. Within offline settings, hospital pharmacies and retail pharmacies demonstrate divergent procurement protocols, reimbursement frameworks, and patient engagement models. Online pharmacy channels, by contrast, capitalize on remote dispensing efficiencies and direct-to-patient delivery innovations. Understanding these channel-specific dynamics provides clarity on how to optimize distribution networks, channel partnerships, and promotional strategies to drive adoption of generic osimertinib.
Highlighting the Distinct Regional Trajectories and Market Drivers Impacting Osimertinib Generic Accessibility Across Americas, EMEA, and Asia-Pacific Health Systems
Regional considerations play a pivotal role in shaping the accessibility and competitive environment for generic osimertinib products. In the Americas, robust regulatory pathways and well-established reimbursement mechanisms have fostered a high level of generic acceptance, while ongoing policy debates around drug pricing continue to influence market entry strategies. Conversely, in Europe, the Middle East & Africa, varied national reimbursement formularies and procurement policies require nuanced market access plans, particularly as tender-based purchasing and reference pricing exert pricing pressure.Meanwhile, the Asia-Pacific region has emerged as a major production hub, with leading manufacturers leveraging economies of scale and government incentives to drive down manufacturing costs. However, this region also presents diverse regulatory requirements and quality assurance standards, necessitating localized registration strategies. These regional nuances underscore the importance of a differentiated market approach, enabling generic developers to align their commercial models with the unique needs and regulatory landscapes of each geography.
Profiling Leading Pharmaceutical Innovators and Generic Manufacturers Shaping the Competitive Landscape of Osimertinib Generics Through Strategic Collaborations and Portfolio Expansion
The competitive landscape for generic osimertinib is dominated by a group of established pharmaceutical firms and specialist generic manufacturers. Leading players have leveraged their global manufacturing networks and regulatory expertise to secure early approvals and capitalize on first-to-file incentives. These companies have also engaged in strategic alliances with active pharmaceutical ingredient suppliers, enhancing their ability to manage cost structures and ensure supply continuity.In addition to large-scale manufacturers, mid-sized and emerging firms have differentiated themselves through targeted licensing agreements and regional partnerships. By focusing on specific markets and forging collaborations with local distributors, these companies aim to capture niche pockets of demand. Across the board, investment in manufacturing scale-up, quality assurance systems, and regulatory affairs capabilities has emerged as a critical factor for success in the generic osimertinib sector.
Strategic Imperatives and Tactical Recommendations for Industry Leaders to Capitalize on Osimertinib Generic Opportunities Amidst Evolving Regulatory and Competitive Landscape
To navigate the evolving generic oncology environment, companies should prioritize strategic partnerships that strengthen supply chain resilience and diversify risk. Establishing long-term agreements with API producers in tariff-exempt regions can stabilize input costs and mitigate regulatory uncertainties. Furthermore, investing in advanced manufacturing technologies will not only enhance production efficiency but also serve as a differentiator in quality-sensitive markets.From a go-to-market perspective, engaging with hospital formulary committees and payer networks through targeted medical education programs can accelerate adoption. Simultaneously, developing patient support initiatives that emphasize affordability and adherence will foster loyalty and drive sustained utilization. Finally, proactive engagement with regulatory agencies to stay ahead of policy changes and leverage expedited pathways will be essential for maintaining competitive positioning in the generic osimertinib landscape.
Robust Research Methodology and Analytical Framework Employed to Deliver Reliable and Actionable Insights on Osimertinib Generic Industry Dynamics
This research leverages a multi-faceted approach, integrating comprehensive secondary research with primary interviews conducted across industry stakeholders, including regulatory experts, manufacturing executives, and oncology practitioners. Data points were triangulated to ensure consistency and accuracy, drawing from publicly available filings, regulatory databases, and peer-reviewed literature. Qualitative insights from expert consultations were supplemented by rigorous validation protocols to confirm emerging trends and competitive dynamics.An analytical framework was developed to segment the market based on dosage strengths and distribution channels, applying custom criteria to capture value chain nuances. Regional analyses were informed by regulatory reviews and stakeholder feedback, ensuring that local market drivers and barriers were accurately represented. Throughout the study, quality control measures, including cross-departmental reviews and peer audits, were employed to maintain the integrity and reliability of the findings.
Conclusive Reflections on Osimertinib Generic Market Evolution and the Strategic Imperatives Guiding Stakeholders Toward Sustained Growth and Innovation
The evolution of the generic osimertinib sector reflects broader shifts in pharmaceutical innovation, regulatory policy, and global supply chain management. As patent exits converge with heightened demand for cost-effective oncology therapeutics, generics are poised to play a transformative role in enhancing treatment accessibility. Key strategic imperatives have emerged around supply chain resilience, regulatory engagement, and differentiated channel strategies.Success in this dynamic environment will hinge on the ability of manufacturers to balance production efficiency with stringent quality standards, while aligning distribution models with the evolving needs of healthcare providers and patients. By embracing collaborative partnerships, investing in advanced manufacturing capabilities, and tailoring approaches to regional complexities, stakeholders can unlock sustainable growth and drive impactful patient outcomes with generic osimertinib products.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Strength
- 40 Mg
- 80 Mg
- Distribution Channel
- Offline Pharmacy
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Offline Pharmacy
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Cipla Limited
- Egis Pharmaceuticals PLC
- Aarti Pharmalabs Ltd.
- Shandong Loncom Pharmaceutical Co., Ltd.
- Alembic Pharmaceuticals Limited
- Bulat Pharmaceutical Co., Ltd.
- Zydus Cadila
- Astrazeneca PLC
- Incepta Pharmaceuticals Ltd.
- Apino Pharma Co., Ltd.
- Shandong Boyuan Chemical Co., Ltd
- MSN Labs
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Osimertinib Generics Market report include:- Cipla Limited
- Egis Pharmaceuticals PLC
- Aarti Pharmalabs Ltd.
- Shandong Loncom Pharmaceutical Co., Ltd.
- Alembic Pharmaceuticals Limited
- Bulat Pharmaceutical Co., Ltd.
- Zydus Cadila
- Astrazeneca PLC
- Incepta Pharmaceuticals Ltd.
- Apino Pharma Co., Ltd.
- Shandong Boyuan Chemical Co., Ltd
- MSN Labs
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
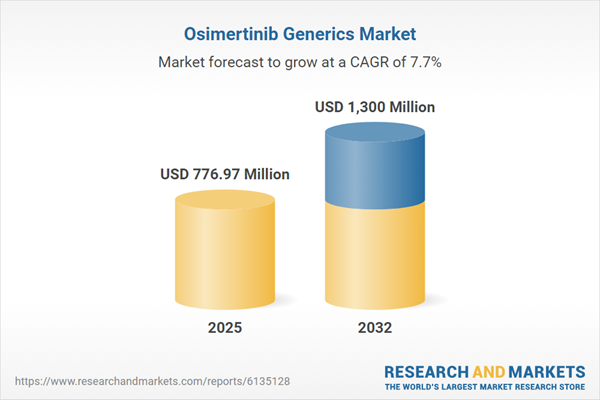
| Estimated Market Value ( USD | $ 776.97 Million |
| Forecasted Market Value ( USD | $ 1300 Million |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |