Speak directly to the analyst to clarify any post sales queries you may have.
The chest seal market is experiencing considerable momentum as technological advances and evolving clinical protocols reshape trauma response solutions. For senior decision-makers, aligning procurement and development strategies with these ongoing market shifts enables stronger positioning and ensures readiness across all settings.
Market Snapshot: Chest Seal Market Growth and Drivers
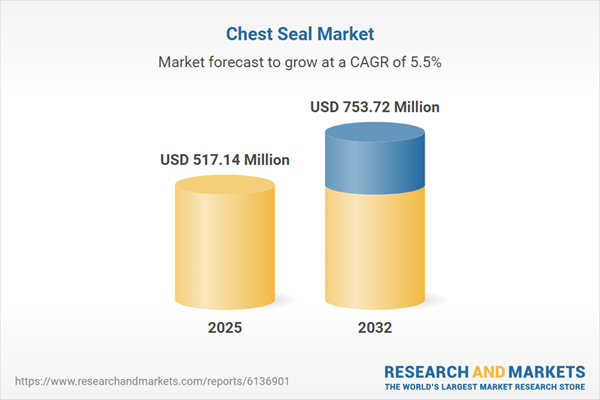
The Chest Seal Market grew from USD 491.30 million in 2024 to USD 517.14 million in 2025. It is expected to continue growing at a CAGR of 5.49%, reaching USD 753.72 million by 2032. This upward trajectory is driven by rising clinical demand, streamlined regulatory pathways, and innovations in material science that bolster the adoption of chest seal products for trauma and emergency care.
Scope & Segmentation of the Chest Seal Market
This report delivers comprehensive analysis, segmenting the chest seal market for executive insights and effective planning.
- Product Types: Non-vented and vented chest seals support various trauma care protocols.
- Material Types: Film and hydrogel-based options influence performance and adherence in challenging scenarios.
- Applications: Battlefield response, emergency treatment, home care, and surgical use each require tailored product solutions.
- End Users: Emergency medical services, home care providers, hospitals, and military or defense operations drive distinct demand profiles.
- Distribution Channels: Hospital and retail pharmacy sales ensure clinical access, while company websites and e-commerce platforms broaden digital procurement avenues.
- Geographies: The Americas, Europe, Middle East & Africa, and Asia-Pacific present diverse regulatory and operational environments affecting market adoption and growth.
- Key Companies: Market leaders include Aero Healthcare, Teleflex Incorporated, North American Rescue, Henry Schein, and Ricci Medical, with each bringing specialized innovations, distribution models, or clinical focus.
Key Takeaways for Senior Decision-Makers
- Chest seal devices are increasingly vital in modern emergency protocols, supporting better trauma outcomes and faster response times.
- Technological progress in hydrogel adhesives, RFID tracking, and packaging design strengthens both device performance and supply chain efficiency.
- Regulatory shifts, particularly streamlined approvals for emergency devices, encourage rapid product iteration and entry into new markets.
- Healthcare procurement and supply chain leaders are focusing on diversified supplier networks and local partnerships to minimize operational disruption from tariffs or regulatory change.
- Digital commerce and education platforms are enabling broader dissemination and better clinician training in both developed and remote regions.
Tariff Impact on Cost Structures and Supply Chains
The introduction of US tariffs in 2025 has led manufacturers to restructure sourcing and reinforce domestic alliances, directly impacting landed costs and encouraging expanded North American production. Lean and agile inventory management methods are being adopted to offset material cost increases. Procurement and finance teams are advised to monitor supplier shifts closely, as new sourcing decisions will influence contract benchmarks and risk management practices.
Methodology & Data Sources
The research leverages qualitative interviews with front-line clinicians and supply chain experts, alongside quantitative analysis from manufacturing records and procurement databases. Peer-reviewed studies and regulatory documents supplement primary findings, ensuring that insights around technology adoption and clinical efficacy are validated and robust.
Why This Report Matters for Decision-Makers
- Provides targeted intelligence to guide resource allocation and portfolio planning across diverse trauma care settings.
- Facilitates risk mitigation and resilience-building by highlighting the influence of tariffs, regulation, and emerging channel preferences on procurement strategy.
- Helps organizations capitalize on innovation trends, from material advances to digitization, supporting improved clinical results while optimizing cost structures.
Conclusion
The chest seal market is evolving quickly, presenting both challenges and opportunities for leaders in healthcare, procurement, and manufacturing. Strategic adaptation grounded in data-driven insights will enable organizations to meet clinical needs and drive sustained market presence.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Chest Seal market report include:- Aero Healthcare
- CELOX Medical Ltd.
- Chinook Medical Gear, Inc.
- EMERGENCY RESPONDER PRODUCTS, LLC
- Medical Devices Inc.
- North American Rescue, LLC
- Rescue Essentials, LLC
- Safeguard Medical Holdco Ltd.
- Tactical Medical Solutions, LLC
- Teleflex Incorporated
- The Seaberg Company Inc.
- Beacon Medical, LLC
- Medline Industries, LP
- WERO GmbH & Co. KG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 517.14 Million |
| Forecasted Market Value ( USD | $ 753.72 Million |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |