Speak directly to the analyst to clarify any post sales queries you may have.
Understanding the Purpose and Scope of the Dexamethasone Implant Executive Summary to Illuminate Key Objectives and Context in Contemporary Ophthalmology
The growing burden of retinal diseases and the need for effective, sustained therapies have elevated the dexamethasone implant as a cornerstone in modern ophthalmic care. With its capacity to deliver corticosteroid medication over an extended period, this implant has reshaped treatment paradigms for conditions that traditionally required frequent intravitreal injections. Stakeholders across the healthcare continuum are now assessing how this innovation can improve patient compliance, enhance visual outcomes, and optimize resource utilization.This executive summary is designed to provide senior leaders and clinical decision makers with a concise yet comprehensive overview of the dexamethasone implant landscape. By synthesizing key trends, market dynamics, regulatory shifts, and competitive activities, it illuminates both the opportunities and challenges that lie ahead. Readers will gain clear insight into the structural forces shaping the market, the implications of recent policy changes, and the strategic actions necessary to maintain a leadership position in this rapidly evolving segment.
Exploring the Fundamental Transformative Shifts Shaping the Dexamethasone Implant Market Landscape Through Technological, Regulatory, and Clinical Advancements
Advancements in drug delivery technology and formulation science have catalyzed a fundamental shift in ophthalmic therapeutics. The dexamethasone implant has capitalized on miniaturized sustained-release mechanisms and biocompatible polymer systems to extend dosing intervals and reduce patient burden. Enhanced precision in intravitreal injection devices, coupled with refined implant compositions, has contributed to improved safety profiles and heightened physician confidence.Simultaneously, regulatory bodies have refined guidelines for intraocular corticosteroid implants, demanding rigorous clinical evidence and post-market surveillance. These evolving standards have encouraged manufacturers to invest in robust safety and efficacy studies, while payers increasingly demand real-world data to support reimbursement decisions. The convergence of clinical innovation and regulatory rigor is redefining treatment pathways and compelling all stakeholders to adapt to a more evidence-driven environment.
Assessing the Comprehensive Implications of United States Tariff Adjustments in 2025 on Supply Chains, Manufacturing Costs, and Adoption Dynamics for Dexamethasone Implants
The implementation of new U.S. tariffs in 2025 has introduced notable pressure on the importation of key components required for dexamethasone implant production. Manufacturers have responded by reassessing global sourcing strategies and exploring domestic supply alternatives to mitigate heightened input costs. These adjustments have prompted a recalibration of pricing frameworks, with companies seeking to balance profitability against the imperative of maintaining patient access.Moreover, distributors and healthcare providers have had to adapt their procurement models to manage the impact of increased import expenses. Some organizations have negotiated volume-based contracts or shifted to alternative distribution channels to preserve cost efficiencies. As reimbursement policies evolve in parallel, stakeholders must navigate a complex interplay of tariff-driven cost increases and payer expectations to sustain market momentum.
Deriving Critical Insights from Segmentation Analysis by Indication, End User, Distribution Channel, and Strength to Guide Strategic Positioning in the Dexamethasone Implant Market
Analysis by indication reveals that diabetic macular edema continues to dominate clinical utilization, with both diffuse macular edema and focal macular edema subcategories driving significant therapeutic demand. Non-infectious posterior uveitis specialists are focusing on intermediate uveitis and panuveitis cases where sustained corticosteroid delivery addresses chronic inflammation with fewer intervention cycles. Retinal vein occlusion remains a key area of interest, particularly in branch retinal vein occlusion and central retinal vein occlusion cohorts where implant efficacy has been demonstrated in vision stabilization.When examining end users, ambulatory surgery centers have emerged as critical settings in both hospital-affiliated facilities and independent centers, reflecting a trend toward outpatient treatment models. Hospital systems-encompassing government and private institutions-continue to integrate implant procedures within broader ophthalmology service lines, while ophthalmic clinics, including multi-specialty eye centers and single-specialty practices, are expanding capacity to meet rising patient volumes. Distribution channel dynamics further underscore the importance of hospital pharmacies across inpatient and outpatient contexts, the growing role of e-commerce platforms in online pharmacies, and the sustained relevance of retail outlets through chain and independent pharmacies. Finally, the core product offering in both 0.7 mg and 1.4 mg strengths ensures clinicians can tailor therapy to disease severity and patient needs.
Unveiling Key Regional Dynamics Across Americas, Europe Middle East & Africa, and Asia Pacific to Highlight Growth Drivers and Adoption Patterns for Dexamethasone Implants
Regionally, the Americas lead in the adoption of dexamethasone implants, driven by high healthcare expenditure, well-established reimbursement frameworks, and robust clinical infrastructure. North American centers of excellence and specialized retina practices have accelerated uptake, positioning the region at the forefront of best practice implementation. Market participants here benefit from integrated care models and transparent regulatory pathways that facilitate timely product launches.In Europe, the Middle East & Africa, heterogeneous reimbursement systems and variable regulatory timelines create a complex environment. While Western European nations display strong implant penetration, emerging healthcare markets in Eastern Europe, the Gulf region, and parts of Africa present both challenges and growth potential. Asia Pacific illustrates rapid expansion, underpinned by increasing retinal disease prevalence, strategic infrastructure investments, and growing private sector involvement. Variations in policy support and provider capacity across Asia Pacific markets will shape differentiated adoption trajectories.
Highlighting Strategic Movements and Competitive Positioning of Leading Companies Shaping Innovation, Collaborations, and Market Penetration in the Dexamethasone Implant Space
Leading companies in the dexamethasone implant space are actively strengthening their portfolios through targeted research collaborations and strategic licensing agreements. These partnerships often align with specialty pharmaceutical firms and academic institutions to accelerate formulation enhancements and expand indications. Concurrently, established pharmaceutical manufacturers are investing in manufacturing scale-up to secure supply continuity and meet rising demand in key geographies.Competitive positioning also reflects ongoing efforts to optimize patient support programs and bolster health economics outcomes. Manufacturers are engaging with payers and providers to generate real-world evidence supporting long-term cost-effectiveness. Additionally, several organizations are exploring next-generation delivery technologies and combination therapies to differentiate their product offerings and sustain market leadership.
Recommending Actionable Strategies for Industry Leaders to Enhance Market Access, Optimize Value Propositions, and Navigate Emerging Opportunities in Dexamethasone Implants
To seize emerging opportunities, industry leaders should explore adaptive pricing strategies that align with evolving reimbursement models and value-based contracting. Strengthening relationships with integrated delivery networks and specialty care centers can enhance market access and streamline patient pathways. Furthermore, forging alliances with domestic component suppliers and logistics providers will mitigate tariff-related cost pressures and reinforce supply chain resilience.Continued investment in next-generation formulations and delivery systems will be critical to maintaining clinical differentiation. Innovators can also integrate digital health solutions to monitor patient adherence and outcomes in real time, thereby generating the evidence required for favorable formulary positioning. Proactive engagement with regulatory bodies and payer organizations will ensure that advocacy for data-driven value assessment remains front and center.
Outlining the Rigorous Research Methodology Combining Primary Interviews, Secondary Data Triangulation, and Analytical Frameworks Underpinning the Dexamethasone Implant Study
This study employed a multi-pronged research approach, combining in-depth interviews with key opinion leaders, industry executives, and clinical specialists to capture firsthand insights. Secondary research encompassed a thorough examination of peer-reviewed publications, clinical trial databases, regulatory filings, and publicly available financial statements to establish a robust evidentiary foundation.Data were cross-validated through rigorous triangulation techniques, ensuring consistency and reliability. Analytical methodologies such as SWOT assessment, value chain analysis, and competitive benchmarking underpinned the evaluation of market dynamics and company strategies. Findings were iteratively reviewed with an expert advisory panel to refine conclusions and reinforce the study's overall integrity.
Concluding Key Takeaways and Strategic Imperatives to Inform Decision Making and Future Directions in the Evolving Dexamethasone Implant Market Landscape
This executive summary has illuminated the key factors shaping the present and future trajectory of the dexamethasone implant market. Technological advancements and regulatory refinements are driving innovation in sustained-release therapies, while tariff changes necessitate strategic supply chain adjustments. Segmentation insights by indication, end user, distribution channel, and strength provide a clear framework for targeted market penetration, complemented by distinct regional growth patterns across the Americas, Europe Middle East & Africa, and Asia Pacific.Competitive dynamics reflect robust investment in research collaborations, manufacturing expansion, and patient support initiatives. By adopting the recommended strategic imperatives-ranging from adaptive pricing and supply chain optimization to digital health integration and regulatory engagement-stakeholders can position themselves to achieve sustainable growth. The insights presented herein serve as a roadmap for informed decision making and decisive action in this vibrant and evolving therapeutic area.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Indication
- Macular Edema
- Diabetic Macular Edema (DME)
- Retinal Vein Occlusion (RVO)
- Non-Infectious Uveitis
- Post-Operative Inflammation
- Macular Edema
- Dosage Form
- 0.35 mg Implants
- 0.7 mg Implants
- Material Type
- Poly(lactic-co-glycolic acid) (PLGA)
- Polycaprolactone (PCL)
- Delivery Route
- Intravitreal
- Subconjunctival
- End Users
- Academic & Research Institutes
- Ambulatory Surgical Centers
- Hospitals
- Ophthalmic Clinics
- Patient Type
- Adult
- Geriatric
- Pediatric
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- AbbVie Inc.
- 3S Corporation
- Santen Pharmaceutical Co., Ltd.
- GNH India Pharmaceuticals Limited.
- Mehan Healthcare Private Limited.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Dexamethasone Implant market report include:- AbbVie Inc.
- 3S Corporation
- Santen Pharmaceutical Co., Ltd.
- GNH India Pharmaceuticals Limited.
- Mehan Healthcare Private Limited.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
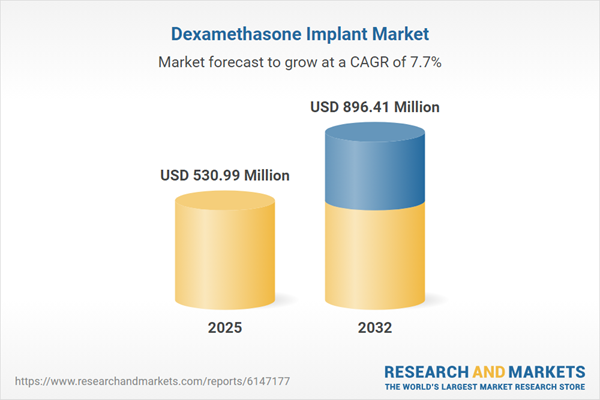
| Estimated Market Value ( USD | $ 530.99 Million |
| Forecasted Market Value ( USD | $ 896.41 Million |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 6 |