Speak directly to the analyst to clarify any post sales queries you may have.
Setting the Stage for the Broad Impact of Recombinant Human Hyaluronidase PH20 Across Therapeutic Modalities and Clinical Applications Worldwide
Recombinant human hyaluronidase PH20 has emerged as a transformative enzymatic agent that addresses critical barriers in drug delivery and tissue permeability by catalyzing the hydrolysis of hyaluronic acid in the extracellular matrix. Leveraging advanced recombinant DNA technology, this enzyme exhibits high specificity and consistent activity, enabling clinicians and pharmaceutical developers to integrate it into a diverse array of therapeutic protocols with confidence. As a result, it has swiftly become a cornerstone in both clinical and commercial settings where rapid dispersion of subcutaneously administered therapies is essential.Across aesthetic procedures, oncology protocols, and subcutaneous infusion therapies, the versatility of hyaluronidase PH20 is driving new paradigms in patient care. Its established safety profile and regulatory approvals have facilitated accelerated adoption, while ongoing research continues to unveil novel applications that promise to enhance efficacy and patient experience. Consequently, stakeholders from drug developers to healthcare providers are closely monitoring technological advancements and evolving best practices to harness the full potential of this enzyme in optimizing treatment outcomes.
Rapid Evolution in Enzyme Therapeutics and Patient Centric Care Driven by Technological Advances and Expanded Applications of Hyaluronidase PH20
Over the past decade, the field of enzyme therapeutics has witnessed a seismic shift driven by innovations in protein engineering and heightened focus on patient centric delivery solutions. Recombinant hyaluronidase PH20 stands at the forefront of this evolution, enabling medical professionals to overcome longstanding challenges in tissue permeability. The integration of this enzyme into established treatment regimens has catalyzed improvements in dermal filler performance, enhanced precision in chemotherapy delivery, and streamlined fluid infusion protocols, collectively advancing patient comfort and therapeutic efficacy.Simultaneously, advancements in formulation science have facilitated the stabilization of this enzyme, extending its shelf life and broadening its applicability across diverse settings. These technological strides are complemented by the growing emphasis on personalized medicine, where hyaluronidase PH20 serves as a critical tool in tailoring dosage and delivery according to individual patient profiles. As a result, industry stakeholders are reevaluating development pipelines, forging collaborative partnerships, and investing in next generation delivery platforms that integrate this enzyme as a core component of innovative treatment modalities.
Assessing the Strategic and Operational Fallout of Upcoming United States Tariff Policy on the Global Supply Chain and Market Dynamics for Recombinant Hyaluronidase PH20
Recent adjustments to import duties and trade policy have introduced new cost considerations for biologic intermediates, notably impacting manufacturers and distributors of recombinant hyaluronidase PH20. By increasing the effective cost of raw materials and finished formulations, these tariffs have prompted supply chain stakeholders to explore alternative sourcing strategies and optimize manufacturing footprints. In turn, companies have accelerated investment in local production capacities, forging regional partnerships to mitigate exposure to tariff fluctuations and ensure continuity of supply.Moreover, organizations are revisiting pricing strategies and value based contracting to counterbalance upward cost pressures without compromising patient access or clinical efficacy. In parallel, regulatory affairs teams have intensified engagement with policymakers to advocate for the recognition of hyaluronidase PH20 as an essential component in therapeutic regimens, seeking tariff exemptions for critical healthcare products. Through these concerted efforts, the industry continues to safeguard both affordability and widespread availability amid a dynamic trade environment.
Unraveling the Complex Landscape of Application Based Uses Product Variants End User Profiles and Distribution Pathways for Hyaluronidase PH20
Insights drawn from the application framework reveal that aesthetic procedures harness the enzyme to enhance dermal filler integration and facilitate wrinkle reduction, while oncology applications leverage its ability to improve chemotherapeutic dispersion and monoclonal antibody tissue penetration. In parallel, subcutaneous drug delivery benefits from accelerated insulin absorption as well as optimized delivery profiles for monoclonal antibodies and peptides. Equally significant, subcutaneous fluid infusion protocols for hypodermoclysis and lymphatic drainage have been streamlined through precise modulation of tissue permeability.When examining product type variations, native formulations continue to serve traditional clinical pathways, whereas stabilized variants are gaining traction in more challenging logistical contexts, underscoring the importance of formulation science in extending shelf stability and broadening usage environments. End user analysis highlights the expanding role of ambulatory surgical centers and clinics in administering enzyme facilitated therapies, complemented by increasing adoption within home care settings and hospitals. Finally, distribution channel patterns indicate that drug distributors and hospital pharmacies remain primary conduits for supply, while online pharmacies and retail pharmacies are emerging as vital touchpoints for convenient access and streamlined procurement.
Exploring Regional Dynamics and Regulatory Environments Shaping the Adoption and Market Integration of Recombinant Hyaluronidase PH20 Across Global Territories
In the Americas, robust regulatory frameworks and established biomanufacturing ecosystems have accelerated the integration of recombinant hyaluronidase PH20 into both clinical research and routine therapeutic protocols. Customer awareness campaigns and reimbursement mechanisms further reinforce its adoption across multiple care settings. Transitioning to the Europe, Middle East and Africa region, evolving regulatory harmonization efforts and strategic collaborations between multinational pharmaceutical firms and regional partners are driving broader market penetration, particularly in oncology and aesthetic medicine.Meanwhile, the Asia-Pacific region is experiencing rapid growth underpinned by expanding healthcare infrastructure, rising patient awareness, and government initiatives to foster local production capabilities. This dynamic environment is characterized by heightened investment in cold chain logistics and quality assurance programs, ensuring that clinicians and patients across emerging markets can reliably access advanced drug delivery solutions. Collectively, these regional patterns emphasize the importance of tailored market entry strategies and regulatory navigation to fully leverage the potential of hyaluronidase PH20 on a global scale.
Profiling Leading Innovators and Established Stakeholders Shaping the Competitive Landscape Through Collaborations Mergers and Strategic Product Developments
Key industry participants have positioned themselves at the vanguard of innovation by prioritizing strategic alliances and targeted research collaborations. Leading biologics developers have supplemented internal pipelines with licensing agreements that enhance their enzyme delivery capabilities, while specialty pharmaceutical firms have expanded their portfolios through merger and acquisition transactions aimed at securing proprietary hyaluronidase technologies. Additionally, emerging players have carved out niche opportunities by focusing on differentiated formulation platforms and regionally tailored manufacturing solutions.Competitive positioning is further defined by investments in scalable production processes, rigorous quality control frameworks, and comprehensive regulatory engagement strategies. By emphasizing end to end supply chain transparency, these organizations are strengthening stakeholder confidence and facilitating smoother market entry in diverse jurisdictions. In aggregate, this constellation of strategic initiatives underscores a concerted effort among top companies to sustain leadership in the evolving enzyme therapeutic landscape and to anticipate future demand shifts through proactive research and development endeavors.
Delivering Strategic Imperatives for Industry Stakeholders to Capitalize on Emerging Opportunities and Mitigate Risks in the Recombinant Hyaluronidase PH20 Sector
To capitalize on the expanding opportunities presented by recombinant hyaluronidase PH20, industry leaders should prioritize the establishment of integrated R&D partnerships that accelerate proof of concept for novel delivery platforms. Proactive engagement with regulatory authorities will facilitate streamlined approval processes and bolster the likelihood of securing tariff exemptions for critical biologic intermediates. In parallel, optimizing manufacturing footprints through regional hubs can mitigate trade policy risks and reduce logistics complexity while ensuring proximity to key end users.Furthermore, organizations should invest in robust health economics studies and real world evidence generation to substantiate the clinical value proposition of hyaluronidase facilitated therapies. Cultivating cross functional teams that encompass supply chain, regulatory affairs, and commercial strategy will improve agility in responding to evolving market dynamics. Finally, leveraging digital engagement tools and educational platforms can enhance practitioner awareness and patient acceptance, ultimately driving adoption rates and reinforcing the enzyme’s position as an indispensable component of contemporary therapeutic regimens.
Detailing a Rigorous Multi Stage Research Framework Incorporating Expert Interviews Comprehensive Data Analysis and Cross Validation for Insight Integrity
This analysis is underpinned by a holistic research framework that integrates both primary and secondary methodologies to ensure comprehensive coverage and insight validity. Primary research comprised structured interviews with senior executives, clinical experts, and distribution partners, complemented by on site visits to manufacturing facilities and infusion centers. These first hand engagements provided nuanced perspectives on operational challenges, adoption drivers, and emerging clinical use cases for enzyme based delivery systems.Secondary research entailed an exhaustive review of regulatory guidelines, peer reviewed journals, patent filings, and industry white papers to contextualize primary findings within prevailing scientific and commercial trends. Quantitative triangulation techniques were applied to reconcile disparate data sources, while rigorous cross validation protocols ensured that conclusions remained robust and actionable. Collectively, this multi stage approach underlines the report’s commitment to delivering an authoritative, data driven perspective on the recombinant hyaluronidase PH20 market landscape.
Synthesizing Critical Insights and Strategic Considerations to Navigate the Evolving Therapeutic and Commercial Terrain of Recombinant Hyaluronidase PH20
As we reflect on the converging forces shaping the trajectory of recombinant hyaluronidase PH20, it becomes evident that this enzyme is at the nexus of innovation in drug delivery and tissue engineering. Technological advancements in formulation stabilization, coupled with the expanding scope of therapeutic applications, reinforce its strategic importance across multiple medical disciplines. Simultaneously, evolving trade policies and regional market dynamics necessitate adaptive strategies that balance cost optimization with patient access imperatives.Looking ahead, the sustained momentum in enzyme based therapeutics will hinge on collaborative efforts among developers, healthcare providers, and regulatory bodies. By harnessing the actionable insights and strategic recommendations presented herein, stakeholders can navigate the complexities of the global marketplace and unlock new avenues for growth. Ultimately, recombinant hyaluronidase PH20 stands poised to redefine the standards of patient centric care and establish new benchmarks for efficacy, safety, and operational excellence within the broader biopharmaceutical ecosystem.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Native Formulation
- Stabilized Formulation
- Route of Administration
- Intramuscular (IM)
- Intravenous (IV)
- Subcutaneous (SC)
- Application
- Aesthetic Procedures
- Dermal Filler Enhancement
- Wrinkle Reduction
- Oncology
- Chemotherapy Enhancement
- Monoclonal Antibody Facilitation
- Subcutaneous Drug Delivery
- Insulin Delivery
- Monoclonal Antibody Delivery
- Peptide Delivery
- Subcutaneous Fluid Infusion
- Hypodermoclysis
- Lymphatic Drainage
- Aesthetic Procedures
- Patient Group
- Adult
- Geriatric
- Pediatric
- End User
- Ambulatory Surgical Centers
- Clinics
- Home Care Settings
- Hospitals
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Johnson & Johnson Services, Inc
- Merck KGaA
- Acro Biosystems, Inc.
- Baxter International Inc.
- Chargen Life Sciences LLP
- Creative BioMart
- Creative Enzymes
- Cusabio Technology LLC
- Eisai Co., Ltd.
- Halozyme, Inc.
- Hangzhou Jiuyuan Gene Engineering Co., Ltd.
- Huonslab Co.,Ltd
- OriGene Technologies, Inc.
- Pfizer Inc.
- Roche Holding AG
- SHANGHAI BAO PHARMACEUTICALS CO.,LTD
- Thermo Fisher Scientific Inc.
- ViiV Healthcare group of companies.
- Zhejiang Hisun Pharmaceutical Co., Ltd.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Recombinant Human Hyaluronidase PH20 market report include:- Johnson & Johnson Services, Inc
- Merck KGaA
- Acro Biosystems, Inc.
- Baxter International Inc.
- Chargen Life Sciences LLP
- Creative BioMart
- Creative Enzymes
- Cusabio Technology LLC
- Eisai Co., Ltd.
- Halozyme, Inc.
- Hangzhou Jiuyuan Gene Engineering Co., Ltd.
- Huonslab Co.,Ltd
- OriGene Technologies, Inc.
- Pfizer Inc.
- Roche Holding AG
- SHANGHAI BAO PHARMACEUTICALS CO.,LTD
- Thermo Fisher Scientific Inc.
- ViiV Healthcare group of companies.
- Zhejiang Hisun Pharmaceutical Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | October 2025 |
| Forecast Period | 2025 - 2032 |
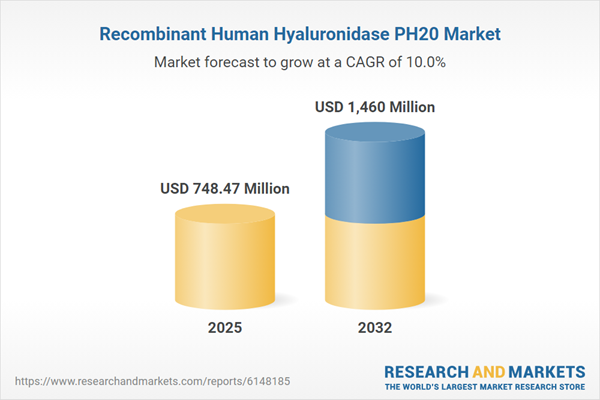
| Estimated Market Value ( USD | $ 748.47 Million |
| Forecasted Market Value ( USD | $ 1460 Million |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |