Speak directly to the analyst to clarify any post sales queries you may have.
Discover the Foundational Drivers and Emerging Context That Are Reshaping the Gynecology Consumables Market and Steering Healthcare Innovation
In recent years, the gynecology consumables domain has emerged as a pivotal segment within the broader healthcare continuum. Driven by demographic shifts that include an aging population and growing emphasis on women's health, the demand for specialized diagnostic and therapeutic tools has intensified. Innovations in material science have yielded advanced plastic and silicone-based consumables that offer improved patient comfort, while stainless steel instruments continue to play a critical role in surgical precision.Furthermore, regulatory landscapes have evolved, fostering rigorous quality and safety protocols that manufacturers must navigate. As healthcare providers strive to optimize clinical outcomes, attention has turned to the seamless integration of consumables within ambulatory surgical centers, diagnostic laboratories, homecare settings, hospitals and specialty clinics. The interplay between reusable and single-use devices has sparked debates around cost efficiency and sterilization processes.
With ongoing advancements in point-of-care diagnostics and minimally invasive procedures, diagnostic consumables such as cervical biopsy punches, endometrial samplers and PAP smear kits have become indispensable. Simultaneously, obstetric accessories like amniotic membrane perforators and disposable birthing kits support safer maternal care. This introduction sets the stage for a comprehensive analysis of market dynamics, transformative shifts and strategic imperatives that define the evolving landscape of gynecology consumables.
Digital connectivity and data analytics are further shaping procurement and utilization patterns, ensuring traceability and informed decision-making. As healthcare ecosystems become more interconnected, stakeholders are prioritizing end-to-end supply chain transparency and embracing online distribution channels alongside traditional offline networks. This executive summary delves into the catalysts propelling change, the cumulative impact of policy shifts, segmentation insights, regional nuances and actionable recommendations for industry leaders aiming to navigate uncertainty and harness emerging opportunities.
Examining Revolutionary Technological Advances Regulatory Evolutions and Patient-Centric Demands Transforming the Gynecology Consumables Landscape
Technological breakthroughs, evolving regulatory frameworks and heightened patient expectations are catalyzing a radical redefinition of the gynecology consumables landscape. Digital platforms now enable real-time inventory management and predictive analytics, reducing stockouts and ensuring critical devices are available when needed. Concurrently, the rise of telemedicine has spurred demand for at-home testing solutions, prompting innovators to develop compact, easy-to-use diagnostic kits that maintain clinical accuracy.Meanwhile, regulatory authorities across key regions have introduced more stringent standards for sterilization, packaging and material biocompatibility. This has led manufacturers to invest in advanced coatings and novel polymers that meet global safety criteria while preserving cost effectiveness. At the same time, patient-centric care models are driving customization, with providers seeking tailored solutions for prenatal monitoring, uterine health assessments and post-operative recovery.
Equally transformative is the shift toward minimally invasive procedures, supported by refined cannulas, trocars and sutures that minimize tissue trauma. As a result, hospital administrators and specialty clinics are prioritizing consumables that can reduce procedure times and enhance patient throughput. Collectively, these shifts underscore an industry in flux, where agility and innovation are paramount for stakeholders aiming to capitalize on emerging opportunities.
Analyzing the Far-Reaching Implications of the 2025 United States Tariff Adjustments on Supply Chains Production Costs and Access for Gynecology Consumables
The implementation of new United States tariffs in 2025 marks a watershed moment for global supply chains in the gynecology consumables arena. Suppliers dependent on imported plastics, silicone and stainless steel face increased duties, prompting a critical reassessment of procurement strategies. Many organizations are now exploring regional sourcing options to mitigate cost volatility, while others are renegotiating long-term contracts to maintain price stability.In response to these changes, contract manufacturers have begun to relocate assembly operations closer to end markets, thereby reducing exposure to cross-border fees. At the same time, some established players are leveraging dual sourcing models, splitting production between domestic and allied international facilities to preserve operational continuity. These structural adjustments have implications for lead times, inventory holdings and capital expenditures, all of which require careful financial modeling.
Moreover, barriers to entry for smaller innovators may increase as import costs squeeze margin profiles. However, the tariff landscape also presents a window of opportunity for local and regional manufacturers to strengthen their foothold by offering competitive pricing and expedited delivery. As stakeholders adapt to this new policy environment, collaboration among suppliers, distributors and healthcare providers will be essential for sustaining supply chain resilience and ensuring uninterrupted patient care.
Delivering Strategic Insights into Material Sterility Usage Application End-User and Distribution Channel Trends Shaping the Gynecology Consumables Sphere
Understanding the full spectrum of segmentation is essential for uncovering nuanced patterns within the gynecology consumables sector. The product type dimension divides the market into consumables and instruments, with consumables further categorized into diagnostic items such as cervical biopsy punches, endometrial samplers and PAP smear kits, obstetric products including amniotic membrane perforators, disposable birthing kits and umbilical cord clamps, and surgical essentials like cannulas, sutures and trocars. Instruments encompass devices such as cervical dilators, curettes, tenaculum forceps, uterine sounds and vaginal speculums, each serving distinct clinical purposes.Material composition has also emerged as a critical factor in both performance and cost considerations. Plastic components are prized for their disposability and hygiene, silicone offers flexibility and patient comfort, and stainless steel remains the standard for durable surgical instruments. Sterility status differentiates products into sterile and non-sterile offerings, influencing handling protocols and storage requirements.
Usage patterns drive further segmentation between reusable and single-use items, reflecting trade-offs between ecological impact and cost efficiency. Applications span diagnostic workflows, continuous monitoring, preventive care, surgical interventions and therapeutic delivery. End users range from ambulatory surgical centers and diagnostic laboratories to homecare settings, hospitals and specialty clinics, each with unique procurement frameworks.
Finally, distribution channels are bifurcated into offline pathways, such as direct sales and distributor networks, and online platforms that facilitate digital procurement and expedited fulfillment. By weaving these segmentation layers together, stakeholders gain a comprehensive vantage point to tailor product portfolios, streamline supply chains and align go-to-market strategies with end-user needs.
Unveiling Distinct Regional Dynamics and Growth Patterns Across the Americas EMEA and Asia-Pacific Markets Fueling Evolution in Gynecology Consumables
Regional characteristics exert a profound influence on supply chain architecture, regulatory compliance and end-user preferences within the gynecology consumables market. In the Americas, robust healthcare infrastructure and widespread adoption of single-use devices converge with growing investment in women's health initiatives, driving demand for minimally invasive surgical tools and advanced diagnostic kits. Suppliers in North America are increasingly prioritizing sustainability credentials and local manufacturing partnerships to meet evolving procurement mandates.Across Europe, the Middle East & Africa, a tapestry of regulatory environments and reimbursement frameworks presents both opportunities and challenges. Western Europe's stringent safety requirements spur innovation in biocompatible materials, while emerging markets in the Middle East and Africa seek affordable, high-quality consumables to support expanding healthcare access. Strategic collaborations and public-private partnerships are becoming common pathways to bridge capability gaps and enhance supply reliability.
In the Asia-Pacific region, dynamic economic growth and rising healthcare expenditure are fueling rapid uptake of both reusable and single-use gynecology products. Domestic manufacturers are scaling production to serve vast populations, while multinational companies establish local R&D hubs to tailor solutions for regional clinical practices. Together, these dynamics illustrate the importance of localized strategies that resonate with the distinct healthcare priorities of each geographic cluster.
Profiling Leading Innovators and Manufacturers Driving Competitive Dynamics and Strategic Partnerships in the Gynecology Consumables Industry Landscape
The competitive landscape of gynecology consumables is defined by a mix of global conglomerates, specialized medical device innovators and agile regional players. Leading firms are extending their portfolios through strategic acquisitions, joint ventures and in-house R&D investments focused on novel materials and integrated diagnostic platforms. At the same time, contract manufacturers are forming alliances with technology startups to co-develop next-generation consumables that leverage miniaturized sensors and digital tracking.Innovation pipelines often emphasize cross-functional collaboration between engineering, clinical and commercial teams, ensuring that new products align with procedural protocols and reimbursement pathways. Many established companies have bolstered their market presence by securing key accounts with large hospital systems and ambulatory surgical centers, thereby gaining valuable feedback loops for continuous product enhancement.
Smaller disruptors are differentiating themselves through niche applications, including telehealth-compatible sampling kits and biodegradable sutures designed to reduce medical waste. Distribution partnerships are evolving, with leading companies integrating e-commerce solutions to supplement traditional distributor networks and accelerate direct-to-clinic fulfillment. By closely monitoring alliances, patent filings and clinical trial outcomes, stakeholders can anticipate competitive moves and identify potential partners for co-innovation.
Empowering Industry Leaders with Actionable Strategies to Enhance Innovation Optimize Supply Chains and Strengthen Market Positioning in Gynecology Consumables
Industry leaders can capitalize on emerging opportunities by adopting a proactive, multi-pronged strategy. First, investing in R&D to develop sustainable materials and digital integration features will address both environmental mandates and the rising demand for connected healthcare solutions. Second, diversifying supply chains through regional production hubs and dual sourcing arrangements can mitigate the impact of geopolitical and tariff-related disruptions.Third, forging alliances with technology providers and healthcare systems enables co-creation of tailored consumables that align with procedural workflows and reimbursement requirements. Fourth, expanding presence in underpenetrated markets via localized manufacturing and culturally attuned marketing efforts will support long-term growth trajectories. Fifth, optimizing channel mix by augmenting traditional distributor relationships with robust online platforms can enhance customer engagement and streamline procurement.
Finally, committing to continuous quality improvement through real-world data collection and patient feedback loops will foster product refinements that elevate clinical outcomes. By executing these recommendations in concert, organizations can strengthen their competitive positioning, drive operational resilience and unlock sustainable growth in the dynamic gynecology consumables landscape.
Detailing Rigorous Research Methodology Data Collection Framework and Analytical Techniques Underpinning the Gynecology Consumables Market Study
This analysis is underpinned by a rigorous research methodology that integrates both primary and secondary data sources. In-depth interviews were conducted with key opinion leaders, procurement specialists and clinical end users to capture firsthand insights into adoption barriers, quality requirements and emerging unmet needs. Complementary desk research involved a comprehensive review of regulatory filings, patent databases and industry publications to validate technological trends and competitive activities.Data triangulation techniques were employed to reconcile discrepancies between quantitative inputs and qualitative observations, ensuring robust conclusions. The study leverages segmentation frameworks covering product type, material, sterility, usage, application, end user and distribution channel to deliver a multidimensional view of market dynamics. Geographic analysis is anchored in macroeconomic indicators, healthcare expenditure patterns and regional policy developments that influence demand and supply factors.
Analytical tools such as SWOT assessments, value chain mapping and scenario planning were applied to identify strategic imperatives and risk factors. Findings were cross-verified through expert panels and peer reviews to uphold analytical rigor. This structured approach provides stakeholders with transparent methodologies and replicable results, enabling confident decision-making in an evolving industry landscape.
Summarizing Key Findings Providing Holistic Perspectives and Identifying Future Horizons for Sustainable Growth and Innovation in Gynecology Consumables
The comprehensive review of gynecology consumables reveals a market at the intersection of innovation, regulation and evolving clinical practice. Technological advances are driving the adoption of more sophisticated diagnostic and surgical tools, while policy shifts such as new tariff regimes are reshaping supply chain strategies. Segmentation analysis underscores the importance of tailoring offerings by product category, material composition, sterility requirements, usage patterns, application scenarios, end-user settings and distribution preferences.Regional insights highlight the divergent priorities across the Americas, Europe, the Middle East & Africa and Asia-Pacific, emphasizing the need for localized go-to-market approaches. Competitive profiling shows that collaboration between established players and disruptive newcomers is accelerating product enhancements and elevating quality standards. Actionable recommendations stress the value of R&D investment, supply chain diversification, strategic partnerships and data-driven performance monitoring.
Ultimately, sustained success in the gynecology consumables arena will depend on an organization's ability to harmonize innovation with operational resilience, meet stringent regulatory demands and respond to the nuanced needs of diverse clinical environments. This conclusion draws together key observations and articulates the strategic paths forward for stakeholders committed to leadership in women's healthcare solutions.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Consumables
- Diagnostic Consumables
- Cervical Biopsy Punches
- Endometrial Samplers
- PAP Smear Kits
- Obstetric Consumables
- Amniotic Membrane Perforators
- Disposable Birthing Kits
- Umbilical Cord Clamps
- Surgical Consumables
- Cannulas
- Sutures
- Trocars
- Diagnostic Consumables
- Instrument
- Cervical Dilators
- Curettes
- Tenaculum Forceps
- Uterine Sounds
- Vaginal Speculums
- Consumables
- Material
- Plastic
- Silicone
- Stainless Steel
- Sterility
- Non-Sterile
- Sterile
- Usage
- Reusable
- Single-use
- Application
- Diagnostic
- Monitoring
- Preventive
- Surgical
- Therapeutic
- End-user
- Ambulatory Surgical Centers
- Diagnostic Laboratories
- Homecare
- Hospitals
- Specialty Clinics
- Distribution Channel
- Offline
- Online
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- 3M Company
- B. Braun Melsungen AG
- Bayer AG
- Boston Scientific Corporation
- Coloplast A/S
- CONMED Corporation
- Cook Medical, Inc.
- Cooper Surgical, Inc.
- FUJIFILM Holdings Corporation
- Gynemed GmbH & Co. KG
- Gynex Corp.
- Hologic, Inc.
- HOYA Corporation
- Johnson & Johnson
- Karl Storz GmbH & Co. KG
- Medtronic plc
- Mölnlycke Health Care AB
- Olympus Corporation
- Paragon Medical, Inc.
- Richard Wolf GmbH
- Smith and Nephew plc
- Stryker Corporation
- Teleflex Incorporated
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Gynecology Consumables Market report include:- 3M Company
- B. Braun Melsungen AG
- Bayer AG
- Boston Scientific Corporation
- Coloplast A/S
- CONMED Corporation
- Cook Medical, Inc.
- Cooper Surgical, Inc.
- FUJIFILM Holdings Corporation
- Gynemed GmbH & Co. KG
- Gynex Corp.
- Hologic, Inc.
- HOYA Corporation
- Johnson & Johnson
- Karl Storz GmbH & Co. KG
- Medtronic plc
- Mölnlycke Health Care AB
- Olympus Corporation
- Paragon Medical, Inc.
- Richard Wolf GmbH
- Smith and Nephew plc
- Stryker Corporation
- Teleflex Incorporated
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
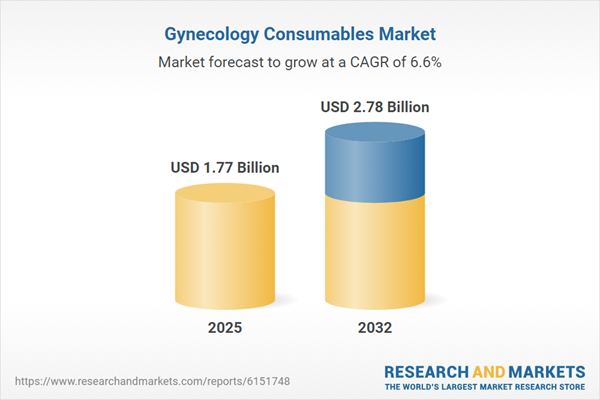
| Estimated Market Value ( USD | $ 1.77 Billion |
| Forecasted Market Value ( USD | $ 2.78 Billion |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |