Speak directly to the analyst to clarify any post sales queries you may have.
Exploring the Rapid Evolution and Growing Importance of At-Home Diagnostic Testing in Modern Healthcare Delivery and Patient Empowerment
The at-home testing landscape has evolved from a niche convenience product into a cornerstone of modern healthcare delivery and patient engagement. Fueled by technological breakthroughs, shifting regulatory frameworks, and heightened consumer demand for accessibility and autonomy, these diagnostics now play an integral role in preventive care, chronic disease management, and rapid response to public health challenges. Beyond simple convenience, the ability for patients to collect samples, perform analyses, and receive results from the comfort of their homes has redefined the parameters of healthcare outreach and continuity of care.As patient centricity becomes a primary objective for providers, payers, and manufacturers alike, at-home testing bridges gaps between clinical settings and everyday life. This confluence of medical-grade accuracy, digital integration, and user-friendly interfaces underscores a shift in healthcare paradigms-one where proactive health monitoring, real-time feedback, and personalized reporting converge. In turn, stakeholders across the healthcare ecosystem must adapt to new levels of data flow, patient education, and quality assurance. With seamless connectivity to telehealth platforms and electronic medical records, at-home diagnostics foster an integrated continuum of care that enhances clinical decision-making and cultivates patient trust.
Consequently, understanding this evolution is crucial for industry leaders seeking to harness at-home testing as a strategic growth driver. This introduction sets the stage for an executive summary that delves into the transformative trends, regulatory influences, segmentation dynamics, regional variations, and actionable imperatives shaping the future of diagnostics outside traditional clinical environments.
Identifying Pivotal Technological, Regulatory, and Consumer Behavior Shifts Reshaping the At-Home Testing Ecosystem and Industry Dynamics
The at-home testing ecosystem is undergoing a fundamental transformation propelled by intertwined technological, regulatory, and consumer behavior shifts. Advancements in microfluidics, biosensor sensitivity, and assay design have enhanced test accuracy and expanded the range of detectable biomarkers. Meanwhile, the integration of smartphone-enabled platforms, cloud-based analytics, and artificial intelligence algorithms has streamlined both user experience and data interpretation, enabling real-time monitoring and predictive health insights. This digital revolution underpins a new era of decentralized diagnostics, where agility and scalability align with precision and accuracy.On the regulatory front, agencies have begun to establish clear pathways for at-home test authorization and market entry. Revised guidance on self-collection procedures, post-market surveillance requirements, and interoperability standards underscores the commitment to patient safety without stifling innovation. At the same time, payers and health systems are reevaluating reimbursement models to incentivize preventive care and remote monitoring, fostering an environment where at-home diagnostics can achieve sustainable adoption.
Consumer attitudes have also shifted dramatically. Growing health literacy, heightened awareness of chronic disease management, and the demand for privacy and convenience have elevated expectations. Individuals now seek seamless experiences that minimize complexity while delivering professional-grade results. In response, manufacturers are adopting human-centered design principles, ensuring clear instructions, intuitive interfaces, and seamless connectivity to digital health ecosystems. Together, these transformative shifts are reshaping both the competitive landscape and the broader healthcare paradigm, driving unprecedented opportunities for stakeholders who can navigate the evolving intersection of technology, regulation, and consumer engagement.
Assessing How the 2025 United States Tariffs Are Altering Supply Chains, Cost Structures, and Competitive Dynamics in the At-Home Testing Market
The implementation of 2025 United States tariffs on imported diagnostic components has introduced a new variable in the at-home testing value chain. Components such as assay reagents, specialized polymers, and electronic modules face increased import costs, prompting manufacturers to reassess supplier networks and to explore alternative sourcing strategies. In many cases, this has triggered a wave of contract renegotiations, with emphasis on geographic diversification and the establishment of nearshore or domestic production capabilities to mitigate exposure to tariff schedules.Supply chain volatility has also compelled organizations to adopt more agile inventory management practices. By combining buffered stockpiles with dynamic demand forecasting methods, companies can reduce lead times and maintain service levels even under fluctuating cost pressures. Strategic partnerships with logistics providers further enable responsive adjustment of freight routes and methods, balancing cost considerations against delivery speed and reliability.
These tariff-driven cost dynamics have influenced pricing strategies and margin structures across the industry. Some market participants have absorbed incremental expenses to preserve volume and market share, while others have passed costs through to end users, accompanied by enhanced value propositions or bundled digital services. In parallel, contract manufacturers located outside the tariff jurisdictions have gained competitive appeal, leading to a redistribution of production volumes and a reshaping of competitive positioning. As stakeholders continue to navigate this evolving tariff landscape, strategic flexibility and supply chain resilience emerge as critical success factors for sustaining growth and profitability.
Unveiling Market Characteristics Through a Detailed Examination of Test Types, Product Offerings, Sample Types, Applications, and Distribution Channels
Understanding the at-home testing market requires an appreciation of its multifaceted segmentation, which influences product development, marketing approaches, and distribution strategies. Tests classified by type reveal a dichotomy between self-collection tests, which enable users to collect samples independently for later analysis, and self-tests that deliver immediate results at home. This distinction affects not only user experience but also regulatory pathways and reimbursement considerations.Product offerings encompass a range of hardware and disposable components, from analyzers designed for repeated use to single-use cassettes, sample collection tubes, and test strips. Each format aligns with specific operational requirements, cost structures, and throughput expectations. Precision analyzers, for example, cater to chronic disease management scenarios, while simple strips and cassettes facilitate rapid diagnostics and mass screening applications.
Additionally, the diversity of sample types-blood, respiratory swabs, saliva, stool, and urine-broadens the scope of health conditions that can be monitored or diagnosed outside clinical settings. Blood-based immunoassays address chronic metabolic conditions, respiratory swabs support infectious disease screening, and saliva or urine tests cover hormone, pregnancy, and genetic assessments. These varied sample matrices demand specialized reagents and collection protocols that directly inform manufacturing complexity and user training requirements.
Applications span blood pressure monitoring, celiac disease screening, cholesterol and triglyceride checks, COVID-19 diagnostics, fecal occult blood profiling, genetic analysis, glucose monitoring, hepatitis C detection, HIV testing, pregnancy confirmation, and thyroid function evaluation. Finally, distribution channels range from traditional brick-and-mortar pharmacies and clinics to online platforms, each offering distinct advantages in reach, speed, and customer engagement. By weaving these segmentation layers into product roadmaps and go-to-market plans, organizations can precisely align offerings with the needs of diverse user cohorts and clinical stakeholders.
Comparative Analysis of At-Home Testing Adoption, Infrastructure Strengths, and Growth Drivers Across the Americas, Europe, Middle East & Africa, and Asia-Pacific
Regional dynamics in the at-home testing sector exhibit distinct patterns driven by healthcare infrastructure, regulatory environments, and consumer adoption trends. In the Americas, mature healthcare ecosystems and high digital literacy have fostered rapid uptake of remote diagnostics, supported by expansive telehealth networks and favorable reimbursement structures. Emerging markets within the region further contribute to growth as public and private stakeholders invest in decentralized testing to alleviate pressure on clinical facilities.In Europe, Middle East & Africa, regulatory harmonization efforts are gradually lowering barriers to cross-border distribution, enabling manufacturers to scale product launches across multiple jurisdictions. Fragmented healthcare systems and varying payer policies, however, necessitate localized market entry strategies. Within the Middle East & Africa, limited infrastructure in certain areas has sparked partnerships between governments and private entities to deploy portable testing solutions in remote communities, yielding important lessons on cost-effective deployment and training.
Asia-Pacific stands out as both a manufacturing hub and an expansive consumer market. Countries with advanced manufacturing capabilities drive innovation in component design and production efficiency, while populous markets with rising health awareness undergird growing demand for monitoring tools. Digital payment infrastructures and e-commerce platforms further accelerate distribution, as consumers seek convenient ordering and rapid delivery. Together, these regional insights illuminate where investment, regulatory advocacy, and strategic partnerships can yield the greatest return and where adaptation to local nuances will determine success.
Highlighting Leading Industry Players' Strategies, Innovation Trajectories, and Collaborative Efforts Driving Competitive Advantage in At-Home Testing
At-home testing has attracted both established diagnostic corporations and agile newcomers, each deploying distinct strategies to capture market share. Leading diagnostics providers are investing heavily in research and development, expanding portfolios to incorporate multiplex assays and digital health platforms. They leverage existing clinical relationships to facilitate adoption among healthcare professionals and integrate test data seamlessly into patient records.Concurrently, entrepreneurial ventures are disrupting traditional models by focusing on user-centric experiences and rapid innovation cycles. These companies often partner with contract manufacturers to accelerate time to market, while prioritizing smartphone connectivity, data analytics, and subscription-based service bundles. Collaborative alliances between startups and technology providers have fostered platforms capable of advanced predictive analytics, enhancing the value proposition for both consumers and clinicians.
Another notable trend involves strategic collaborations with pharmaceutical firms, insurers, and healthcare systems to co-create tailored solutions for chronic disease management and wellness monitoring. By embedding at-home diagnostics into broader care pathways, these partnerships generate recurring revenue streams and reinforce patient engagement. As competition intensifies, proprietary assay technologies and integrated software ecosystems are emerging as key differentiators. Stakeholders that can synchronize clinical efficacy, regulatory compliance, and seamless user experiences will lead the next wave of growth in this dynamic landscape.
Strategic Imperatives and Best Practice Recommendations for Market Participants to Capitalize on Emerging Trends and Secure Long-Term Growth
To thrive in the evolving at-home testing domain, market participants must adopt a suite of strategic imperatives that align with technological, regulatory, and consumer landscapes. First, integrating digital health capabilities-including secure data transmission, real-time monitoring dashboards, and AI-driven insights-can enhance user engagement and support differentiated service offerings. By leveraging these tools, organizations can transition from traditional product vendors to holistic care partners.Second, diversifying supply chain footprints through geographic expansion and dual sourcing models will mitigate tariff exposure and component shortages. Establishing flexible manufacturing networks that can pivot between domestic and international facilities ensures continuity of supply under shifting trade policies. In parallel, engaging logistics providers with advanced tracking and contingency planning capabilities will preserve distribution reliability.
Third, proactive regulatory engagement is essential. By collaborating with authorities to shape emerging guidelines on self-testing, data privacy, and digital interoperability, companies can secure expedited approval pathways and favorable market access conditions. Aligning reimbursement models with payers early in product development fosters recognition of at-home testing as a reimbursable healthcare service.
Finally, cultivating cross-sector partnerships with technology firms, healthcare systems, and patient advocacy groups will deepen market penetration and build trust. Co-development initiatives can yield tailored solutions for specific patient populations, while joint education campaigns enhance user adoption. Through these actionable recommendations, industry leaders can capitalize on emerging trends, navigate complexity, and establish sustainable competitive advantage.
Transparency into the Rigorous Research Framework, Data Collection Techniques, and Analytical Approaches Underpinning This At-Home Testing Study
This study employs a comprehensive research methodology grounded in both primary and secondary data collection, ensuring robust validation and triangulation of insights. Secondary research encompassed a review of regulatory publications, clinical journals, patent filings, and industry white papers to map technological advancements and policy developments. Data from trade associations, government agencies, and academic institutions provided context on sample type usage, distribution channel dynamics, and regional healthcare infrastructures.Primary research consisted of in-depth interviews with stakeholders across the at-home testing ecosystem, including manufacturing executives, regulatory specialists, clinical laboratory leaders, and digital health innovators. These discussions illuminated real-world challenges in supply chain resilience, market access, and consumer engagement. Insights were further enriched by structured surveys distributed to healthcare providers and end users, capturing attitudes toward self-collection protocols and digital integration features.
Analytical approaches incorporated cross-segment comparisons, thematic coding of qualitative data, and scenario planning to assess the impact of trade policy shifts and technological disruptions. Each data point underwent rigorous quality checks, and findings were synthesized into a cohesive framework that underpins the segmentation, regional, and company insights presented in this executive summary. The methodology's transparency ensures that decision-makers can appraise the reliability of conclusions and replicate core analytical steps in future research endeavors.
Synthesizing Key Findings and Strategic Takeaways to Inform Stakeholders' Decisions in the Evolving At-Home Testing Landscape
In synthesizing key findings, it becomes clear that at-home testing stands at the confluence of technological innovation, shifting regulatory landscapes, and evolving consumer expectations. The convergence of advanced assay development, digital health integration, and agile supply chain strategies has created fertile ground for both established players and disruptors. Rather than a static market, diagnostics outside clinical settings represent a dynamic ecosystem where success hinges on adaptability, strategic collaboration, and relentless focus on user experience.Regional variations underscore the need for nuanced approaches. While mature markets benefit from established telehealth infrastructures and favorable reimbursement policies, emerging regions offer untapped potential through infrastructure partnerships and localized product adaptations. Moreover, the competitive arena is defined not only by assay accuracy or cost efficiency but also by seamless integration into broader care pathways and data ecosystems.
Looking ahead, regulatory alignment, customer-centric design, and data-driven service models will dictate the pace of adoption. Stakeholders that anticipate policy changes, invest in end-to-end digital solutions, and cultivate resilient supply chains will capture early mover advantages. Ultimately, the promise of at-home testing lies in its ability to decentralize healthcare, empower patients, and alleviate burdens on traditional clinical settings. This conclusion reaffirms the strategic imperative for industry leaders to act decisively and collaboratively in shaping a future where diagnostics are accessible, accurate, and integrated into everyday life.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Type
- Self Collection Tests
- Self Tests
- Product Offering
- Analyzers
- Cassette
- Sample Collection Tubes
- Test Strips
- Sample Type
- Blood
- Respiratory Swab
- Saliva
- Stool
- Urine
- Application
- Blood Pressure Test
- Celiac Disease Test
- Cholesterol & Triglycerides Tests
- COVID-19 Test
- Fecal Occult Blood Test
- Genetic Test
- Glucose Monitoring
- Hepatitis C Test
- HIV Testing
- Pregnancy Test
- Thyroid Test
- Distribution Channel
- Offline
- Online
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Abbott Laboratories
- B. Braun Melsungen AG
- ACON Laboratories, Inc.
- Bionime Corporation
- Cardinal Health Inc
- DxTerity Diagnostics, Inc.
- Eugene Labs
- Everly Health, Inc
- F. Hoffmann-La Roche Ltd
- Fulgent Genetics
- iHealth Labs Inc.
- LetsGetChecked
- Mankind Pharma Ltd.
- OraSure Technologies, Inc.
- Pfizer Inc.
- PHC Holdings Corporation
- QuidelOrtho Corporation
- RxHomeTest
- Siemens Healthineers AG
- SiPhox, Inc.
- Thriva Ltd
- TouchBio
- Viome Life Sciences, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this At-Home Testing Market report include:- Abbott Laboratories
- B. Braun Melsungen AG
- ACON Laboratories, Inc.
- Bionime Corporation
- Cardinal Health Inc
- DxTerity Diagnostics, Inc.
- Eugene Labs
- Everly Health, Inc
- F. Hoffmann-La Roche Ltd
- Fulgent Genetics
- iHealth Labs Inc.
- LetsGetChecked
- Mankind Pharma Ltd.
- OraSure Technologies, Inc.
- Pfizer Inc.
- PHC Holdings Corporation
- QuidelOrtho Corporation
- RxHomeTest
- Siemens Healthineers AG
- SiPhox, Inc.
- Thriva Ltd
- TouchBio
- Viome Life Sciences, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
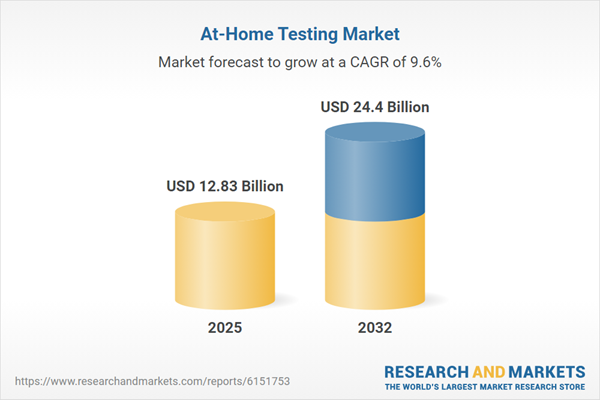
| Estimated Market Value ( USD | $ 12.83 Billion |
| Forecasted Market Value ( USD | $ 24.4 Billion |
| Compound Annual Growth Rate | 9.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |