Market growth is supported by the rising demand for quick and easy testing outside traditional labs, especially in emergency rooms and clinics. The growing number of infectious disease cases and the need for fast results are pushing the use of point-of-care molecular tests. New technologies, such as portable PCR machines and simple sample processing tools, make these tests more accurate and easier to use. In addition, the focus on faster testing after the COVID-19 pandemic is helping the market grow further.
Moreover, point-of-care molecular diagnostics in primary care settings cover a wide range of uses, from basic glucose tests to more complex blood clotting checks. Many clinics across the U.S. are moving away from traditional lab-based testing to point-of-care methods, which help reduce delays linked to sample handling and transport. This switch allows for quicker decisions during patient visits, leading to faster results, lower costs, and improved care.
In addition, the growing elderly population is expected to support the growth of the U.S. point of care molecular diagnostics industry. Based on the U.S. National Cancer Institute’s SEER Database, 38% of women and 43% of men are likely to develop cancer during their lifetime. Nearly two-thirds of all new cancer cases are found in people aged 65 and older, showing that aging increases vulnerability to cancer. Molecular diagnostics play a key role in managing cancer, infectious diseases, and heart conditions, making them an essential part of timely diagnosis and treatment in older adults.
Furthermore, market players are actively developing new point-of-care molecular testing products to address growing diagnostic needs. In April 2023, Curative, Inc., based in Los Angeles, announced the spin-off of Sensible Diagnostics to commercialize a desktop PCR testing platform. This system is designed to deliver lab-quality results in approximately 10 minutes, at a cost similar to lateral flow antigen tests. It is intended for use in retail clinics, urgent care centers, and other near-patient environments.
In August 2023, Sensible Diagnostics received a NIH RADx Tech award of approximately USD 1 million to support the development of a multiplex respiratory viral panel for point-of-care use. These efforts reflect growing investment in rapid, accurate molecular testing solutions that support timely diagnosis and improve clinical workflows across decentralized care settings.
U.S. Point of Care Molecular Diagnostics Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2021 to 2033. For this report, the analyst has segmented the U.S. point of care molecular diagnostics market report based on application, technology, test location, and end-use:Technology Outlook (Revenue, USD Million, 2021 - 2033)
- PCR-based
- Genetic Sequencing-based
- Hybridization-based
- Microarray-based
Application Outlook (Revenue, USD Million, 2021 - 2033)
- Infectious Diseases
- HIV POC
- Clostridium difficile POC
- HBV POC
- Pneumonia or Streptococcus associated infections
- Respiratory syncytial virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and drug-resistant TB POC
- HSV POC
- Other Infectious Diseases
- Oncology
- Hematology
- Complete blood count (CBC)
- Prothrombin time (PT)
- Partial Thromboplastin Time (PTT)
- Others
- Prenatal Testing
- Endocrinology
- Other Applications
Test Location Outlook (Revenue, USD Million, 2021 - 2033)
- OTC
- POC
End-use Outlook (Revenue, USD Million, 2021 - 2033)
- Decentralized Labs
- Hospitals
- Home-care
- Assisted Living Healthcare Facilities
- Others
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned
- QIAGEN
- Danaher
- Thermo Fisher Scientific, Inc.
- BD
- F. Hoffman-La Roche AG
- Charles River Laboratories
- Quest Diagnostics Incorporated
- Bio-Rad Laboratories, Inc.
- Hologic Inc.
- Agilent Technologies, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | July 2025 |
| Forecast Period | 2024 - 2033 |
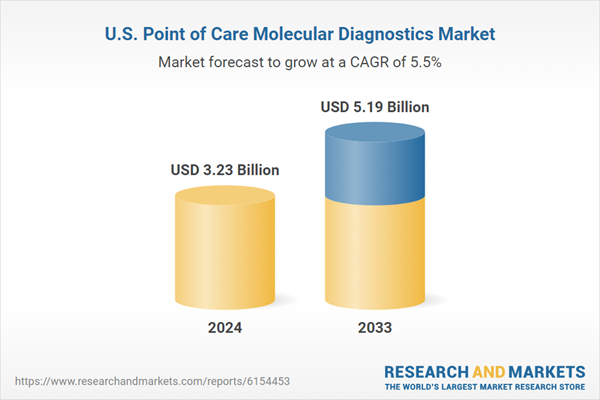
| Estimated Market Value ( USD | $ 3.23 Billion |
| Forecasted Market Value ( USD | $ 5.19 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |