The current scenario for global research and development activities and the need for several new treatment options have also led to the adoption of fast-track clinical trials. Thus, the factors above are estimated to offer new avenues for the clinical trials market growth. Favorable government support and initiatives are another aspect boosting the market growth potential. For instance, the WHO launched Solidarity, an international clinical trial to determine effective treatment against COVID-19. It includes comparing four treatment options against the standard of care to evaluate their effectiveness against the coronavirus.
Government initiatives and investments by biotechnology and pharmaceutical firms are driving medical research activities. Combined with technological advancements, these factors are expected to propel market growth. For example, in October 2023, the Advanced Research Projects Agency for Health (ARPA-H), a U.S. Department of Health and Human Services (HHS) agency, announced plans to enhance the country's ability to conduct clinical trials rapidly, safely, and equitably. This initiative aims to promote technological advancements and insights to establish a robust national clinical trial infrastructure, thereby fostering the adoption of CTMS and strengthening the market growth.
Moreover, market players are introducing tailored solutions or optimizing existing ones to cater to the requirements of Decentralized Clinical Trials (DCTs). For instance, Cloudbyz offers a cloud-based CTM solution with features like ePRO, remote monitoring & SDV, eConsent, and eCRF, supporting virtual trials. Parexel International Corporation, a key player, has conducted over 250 fully virtual or hybrid DCTs and has experience with various remote patient engagement strategies incorporated into trials.
Increasing R&D investments by life science and medical device companies, coupled with the growing prevalence of acute & chronic disorders, are driving the surge in clinical trials. This trend is expected to boost the demand for solutions like CTMS, facilitating efficient management of diverse clinical trials and ultimately improving patient outcomes.
U.S. Clinical Trial Management System Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, the analyst has segmented the U.S. clinical trial management systems market report based on solution type, component, delivery mode, and end use:Solution Type Outlook (Revenue, USD Million, 2018 - 2030)
- Enterprise
- Site
Component Outlook (Revenue, USD Million, 2018 - 2030)
- Software
- Services
Delivery Mode Outlook (Revenue, USD Million, 2018 - 2030)
- Web & Cloud Based
- On Premise
End use Outlook (Revenue, USD Million, 2018 - 2030)
- Pharmaceutical and Biotechnology Firms
- Medical Device Firms
- CROs & Others
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- IQVIA, Inc.
- Medidata (Dassault Systèmes)
- Oracle
- DATATRAK International, Inc.
- Clario
- SimpleTrials
- Calyx (formerly Parexel Informatics)
- RealTime Software Solutions, LLC
- Laboratory Corporation of America Holdings
- Veeva Systems
- Wipro Limited
- MedRhythms
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 100 |
| Published | July 2025 |
| Forecast Period | 2024 - 2030 |
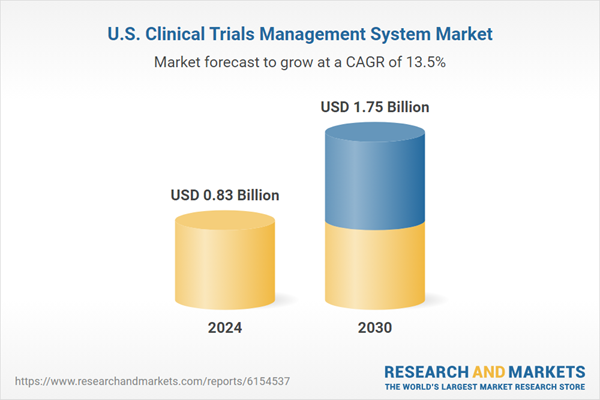
| Estimated Market Value ( USD | $ 0.83 Billion |
| Forecasted Market Value ( USD | $ 1.75 Billion |
| Compound Annual Growth Rate | 13.5% |
| Regions Covered | United States |
| No. of Companies Mentioned | 12 |