In addition, the growing volume of sample testing has led to notable improvements in data management and sample preparation, supporting more efficient laboratory workflows and broader test availability. These enhancements are helping laboratories reduce turnaround times, improve diagnostic accuracy, and handle rising demand across various healthcare settings. Integration of automated systems and digitized reporting tools is also strengthening operational capacity, enabling faster delivery of results and better coordination between clinicians and lab professionals.
The market is also witnessing growing interest from new entrants aiming to capitalize on the sector’s strong potential. Driven by the sector’s lucrative prospects, new entrants are actively investing in the U.S. For instance, in February 2023, Dutch biotech firm Detact Diagnostics established a new laboratory at Keene State College under a two-year rental agreement. Similarly, in May 2021, Neuberg Diagnostics launched a U.S. lab focused on next-generation sequencing (NGS) and molecular diagnostics-key areas for precision medicine and early disease detection. These investments reflect the expanding scope of specialized testing and the increasing role of innovation in shaping future clinical laboratory services.
Moreover, the evolving regulatory environment and focus on value-based care are further influencing the development of clinical laboratory services in the U.S. Laboratories are increasingly aligning with quality standards set by organizations such as CLIA and CAP to maintain compliance and enhance credibility. At the same time, payers' emphasis on cost-effectiveness and clinical utility encourages labs to adopt more targeted testing strategies. This shift reinforces the need for evidence-backed diagnostics that support clinical decision-making and contribute to improved patient outcomes and optimized healthcare spending.
Prior to the COVID-19 pandemic, over 13 billion clinical tests were conducted annually across more than 200,000 CLIA-certified laboratories, highlighting the essential role of diagnostics in the healthcare system. This infrastructure plays a key role in managing chronic conditions, with nearly 60% of the U.S. population living with at least one chronic disease as of 2022, according to the National Association of Chronic Disease Directors. The clinical demand for routine and specialized testing continues to rise with the country’s aging population and the shift toward preventive care.
The pandemic further underscored the critical role of diagnostics, with over 1.17 billion COVID-19 tests performed in the U.S. by March 2023. This surge in testing capacity has strengthened public health preparedness and accelerated investment in lab infrastructure. New entrants are actively tapping into the market’s potential, such as Detact Diagnostics, which established a laboratory at Keene State College in February 2023, and Neuberg Diagnostics, which launched a U.S. lab for next-generation sequencing and molecular diagnostics in May 2021. In addition, ProPhase Labs introduced DNA Complete, Inc. in November 2024, a direct-to-consumer DNA testing platform reflecting the market's growing orientation toward personalized diagnostics. Rising cancer incidence-projected to exceed 2 million new cases in 2024-further reinforces the ongoing demand for advanced laboratory services, particularly in oncology-focused testing.
U.S. Clinical Laboratory Services Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest trends in each of the sub-segments from 2021 to 2033. For this report, the analyst has segmented the U.S. clinical laboratory services market report based on test type,application, and service provider:Test Type Outlook (Revenue in USD Million, 2021 - 2033)
- Genetic Testing
- Clinical Chemistry
- Routine Chemistry Testing
- Therapeutic Drug Monitoring Testing
- Endocrinology Chemistry Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Medical Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Immunology Testing
- Cytology Testing
- Drug of Abuse Testing
- Other Esoteric Tests
Service Provider Outlook (Revenue in USD Million, 2021 - 2033)
- Hospital-Based Laboratories
- Stand-Alone Laboratories
- Clinic-Based Laboratories
Application Outlook (Revenue in USD Million, 2021 - 2033)
- Bioanalytical & Lab Chemistry Services
- Toxicology Testing Services
- Cell & Gene Therapy Related Services
- Preclinical & Clinical Trial Related Services
- Drug Discovery & Development Related Services
- Other Clinical Laboratory Services
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Laboratory Corporation of America Holdings (LabCorp)
- QIAGEN NV
- Eurofins Scientific SE
- Quest Diagnostics Incorporated
- OPKO Health, Inc.
- Siemens Medical Solutions USA, Inc.
- NeoGenomics Laboratories.
- Fresenius Medical Care.
- ARUP Laboratories.
- Sonic Healthcare
- Charles River Laboratories International, Inc.
- SYNLAB International GmbH
- Mayo Clinic Laboratories
- Unilabs
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | July 2025 |
| Forecast Period | 2024 - 2033 |
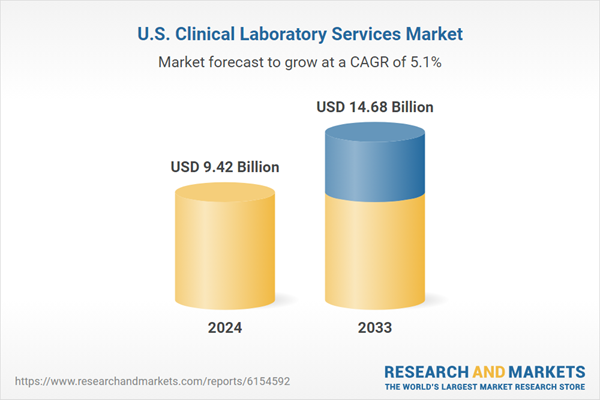
| Estimated Market Value ( USD | $ 9.42 Billion |
| Forecasted Market Value ( USD | $ 14.68 Billion |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | United States |
| No. of Companies Mentioned | 14 |