Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive orientation to aflibercept's clinical roles, commercial pathways, and strategic touchpoints across ophthalmology and oncology treatment landscapes
Aflibercept sits at the intersection of advanced biologic engineering and high-impact ophthalmology therapeutics, with clinical applications that span complex retinal vascular diseases and oncology formulations. Its mechanism as a soluble decoy receptor for vascular endothelial growth factor (VEGF) has underpinned durable therapeutic responses in wet age-related macular degeneration and diabetic retinal disease, while a distinct formulation finds utility in systemic oncology regimens. This executive summary synthesizes cross-cutting developments that are reshaping competitive positioning, care delivery patterns, regulatory interactions, and supply chain strategies for stakeholders across development, manufacturing, and clinical practice.Throughout this analysis, emphasis is placed on actionable interpretation rather than raw forecasts. The narrative traces how evolving dosing paradigms, delivery formats, and biosimilar entrants interact with payer pressures and site-of-care shifts. By integrating clinical trendlines, regulatory milestones, and commercial initiatives, the introduction establishes a foundation for subsequent sections that examine structural shifts, policy impacts, segmentation nuances, regional differentials, and recommended responses. Consequently, readers can align programmatic priorities with operational levers that influence adoption, adherence, and long-term value capture.
Analysis of pivotal clinical, commercial, and delivery innovations that are reconfiguring aflibercept adoption, access mechanisms, and competitive differentiation
The therapeutic landscape for aflibercept is undergoing transformative shifts driven by scientific refinement, care delivery optimization, and competitive dynamics. Advances in dosing strategies and extended-interval regimens are prompting re-evaluation of clinic throughput and patient monitoring protocols, while parallel work on formulation and delivery is stimulating interest in pre-filled systems and alternative administration techniques. At the same time, biosimilar development and lifecycle management initiatives are introducing new layers of competitive pressure, prompting incumbent developers to amplify differentiation through real-world evidence, label expansions, and patient support programs.Transitioning from clinical to operational impacts, these shifts influence where care is delivered and by whom. Ambulatory surgical centers and ophthalmology specialty clinics are adapting to higher volumes and evolving injection frequencies, and hospitals are recalibrating resource allocation for complex retinal procedures. Additionally, payer and procurement stakeholders increasingly emphasize value-based contracting and outcomes-based reimbursement, compelling manufacturers to demonstrate tangible improvements in patient-reported outcomes and durability of effect. Together, these forces are recalibrating strategic priorities and creating opportunities for organizations that can integrate clinical innovation with pragmatic commercialization and supply chain resilience.
Thoughtful assessment of how United States tariff adjustments projected for 2025 could alter sourcing economics, domestic manufacturing incentives, and competitive cost pressures
Anticipated changes to United States tariff frameworks and associated trade policies in 2025 are likely to exert measurable pressure across pharmaceutical manufacturing and distribution chains, with cascading implications for aflibercept stakeholders. Increased duties on imported active pharmaceutical ingredients, specialized biologics components, and packaging materials can raise input costs and compress margins unless mitigated by operational adjustments. Moreover, tariff-driven incentives to onshore certain manufacturing steps could accelerate domestic capacity investments, but such moves require lead time and capital commitments that favor larger, vertically integrated players.In parallel, potential tariff shifts will influence contract manufacturing relationships and the geographic logic of supplier selection. Companies may respond by diversifying supplier bases, negotiating long-term raw material agreements with tariff pass-through clauses, or pursuing strategic vertical partnerships that stabilize supply and control costs. From a commercial perspective, these changes could increase the attractiveness of biosimilar entrants that localize manufacturing or leverage alternative sourcing strategies, thereby intensifying competitive dynamics. Consequently, executives should view tariff adjustments as a catalyst for revisiting sourcing strategies, cost-to-serve models, and inventory management practices to preserve product availability and maintain predictable access for treating clinicians and patients.
In-depth segmentation synthesis revealing how product types, delivery formats, administration routes, clinical applications, care settings, and channels shape strategy
Segment-level dynamics reveal differentiated imperatives for product development, manufacturing, and commercial engagement. Within product type segmentation, the landscape spans established biologic formulations and the emergence of biosimilars, each demanding distinct lifecycle strategies; innovators typically focus on incremental clinical differentiation and integrated services, whereas biosimilar developers prioritize manufacturing scale, regulatory clarity, and access agreements. When considering dosage type segmentation, the availability of pre-filled syringes versus vials reshapes clinician workflow and patient convenience; pre-filled systems can reduce preparation variability and support higher throughput in specialty clinics, while vials offer flexibility for multi-dose practices and cost-sensitive procurement.Route of administration segmentation further delineates development priorities: intravitreal injection remains the primary modality for retinal disease with concentrated needs around sterility, delivery precision, and patient comfort, whereas systemic intravenous formulations intersect with oncology treatment centers and different payer considerations. Application segmentation highlights varied clinical endpoints and stakeholder expectations across diabetic macular edema, diabetic retinopathy, myopic choroidal neovascularization, retinal vein occlusion, and wet age-related macular degeneration, each requiring tailored evidence packages and provider education. End user segmentation underscores the operational differences among ambulatory surgical centers, hospitals, and ophthalmology centers with respect to staffing, storage capabilities, and procurement cycles. Finally, distribution channel segmentation-offline versus online-affects how products reach clinicians and patients, influencing inventory models, cold chain requirements, and digital patient engagement initiatives. Together, these layered segment perspectives inform differentiated commercialization plans and product design choices.
Regional differentiation analysis showing how regulatory complexity, procurement frameworks, and service capacity drive tailored commercial and operational approaches
Regional variation creates distinct strategic priorities across the Americas, Europe, Middle East & Africa, and Asia-Pacific, driven by divergent regulatory frameworks, reimbursement architectures, and healthcare delivery models. In the Americas, concentrated centers of clinical excellence and integrated payer-provider arrangements place a premium on demonstrating longitudinal outcomes and cost-effectiveness to secure coverage and formulary positioning. Consequently, lifecycle programs that combine robust post-approval evidence generation with targeted access initiatives tend to perform well in this environment.Moving to Europe, Middle East & Africa, stakeholders must navigate heterogeneous regulatory pathways, variable procurement mechanisms, and differing levels of infrastructure for cold chain and specialty care. This region often rewards flexible distribution models and locally adapted pricing strategies, while public procurement processes can favor cost-competitive entrants that meet strict quality standards. In the Asia-Pacific region, rapid growth in specialized ophthalmology services is accompanied by expanding capacity for local manufacturing and rising demand for efficient care delivery. Market participants in this geography typically benefit from partnerships with regional manufacturers, investments in clinician training, and culturally tailored patient support services. Across all regions, translating clinical value into accessible care hinges on aligning evidence generation with local regulatory expectations and payer priorities, while operational adaptations ensure reliable product supply and adoption.
Strategic and operational company-level perspectives highlighting how innovators, biosimilar entrants, and partners are shaping competitive positioning and supply continuity
Companies operating in the aflibercept ecosystem are pursuing a mix of defensive and offensive strategies to protect and expand their positions. Incumbent innovators focus on extending clinical indications, optimizing dosing intervals, and reinforcing service offerings such as patient support, adherence programs, and clinician education to preserve differentiation. Concurrently, organizations building biosimilar and alternate biologic programs emphasize manufacturing efficiency, regulatory pathway optimization, and targeted market entry where access barriers are lower. Across these approaches, strategic partnerships and licensing agreements remain central tools for sharing development cost, accelerating geographic rollouts, and aligning commercial capabilities with local market needs.Operationally, leading players are investing in scalable sterile manufacturing, enhanced quality systems, and cold chain logistics to ensure continuity of supply and to meet increasingly stringent regulatory expectations. Market-facing tactics include value-based contracting pilots, outcomes tracking to substantiate long-term benefits, and digital engagement platforms that improve adherence and monitor safety. From a corporate perspective, mergers, acquisitions, and selective divestitures are being used to consolidate capabilities, acquire complementary pipelines, or rationalize portfolios in response to evolving competitive pressures. These company-level maneuvers collectively shape the competitive contours of the aflibercept landscape and determine which organizations can sustain durable access and commercial success.
Practical and prioritized steps for leaders to strengthen evidence, secure supply chains, optimize delivery formats, and align commercial incentives for sustained growth
To translate insight into sustainable advantage, industry leaders should prioritize a coordinated set of actions across development, commercial, and operational domains. First, invest in real-world evidence generation that aligns clinical endpoints with payer value frameworks and supports differentiated reimbursement discussions. Second, optimize product format strategy by evaluating the trade-offs between pre-filled syringes and vials for specific care settings, thereby enhancing clinician efficiency and patient experience. Third, strengthen supply chain resilience through supplier diversification, strategic inventory buffers, and selective onshoring of high-risk inputs to mitigate exposure to tariff and trade disturbances.Furthermore, pursue targeted stakeholder engagement programs that educate clinicians and payers about optimized dosing regimens and long-term outcomes, while deploying tailored patient support initiatives to reduce treatment attrition. Emphasize partnerships with ambulatory surgical centers, hospitals, and specialty ophthalmology clinics to co-create service models that streamline administration and follow-up care. Finally, consider commercial contracting innovations-such as outcomes-linked agreements and flexible pricing models-that align incentives across manufacturers, providers, and payers. Taken together, these actions balance near-term operational stability with longer-term differentiation and access objectives.
Robust mixed-methods research design combining expert interviews, regulatory and clinical evidence mapping, supply chain assessments, and iterative validation to ensure actionable findings
The research methodology underpinning this analysis combines qualitative and quantitative rigor to deliver defensible insights and pragmatic recommendations. Primary inputs included structured interviews with clinical experts, supply chain managers, payer representatives, and commercial leaders to validate trends and surface execution risks. Secondary research encompassed regulatory filings, peer-reviewed clinical literature, clinical trial registries, and publicly available commissioning guidance to map evidence requirements and approval pathways. Data triangulation was applied to reconcile divergent inputs, while scenario analysis stressed operational and policy sensitivities that could materially affect access and cost dynamics.Validation steps included workshops with key opinion leaders and iterative reviews by subject-matter experts in ophthalmology, biologic manufacturing, and market access. Where appropriate, supply chain mapping and supplier capability assessments informed risk prioritization, and real-world evidence frameworks guided recommendations for post-authorization studies. Throughout, emphasis was placed on transparency of assumptions and on linking methodological choices directly to the needs of commercial, clinical, and regulatory stakeholders to ensure the outputs are actionable and relevant to decision-makers.
Concise synthesis of evolving clinical, commercial, and operational factors that will determine which strategic approaches deliver resilient access and long-term value
In conclusion, aflibercept's strategic landscape is defined by converging clinical innovation, shifting care delivery paradigms, and intensifying commercial competition. Extended dosing regimens, evolving delivery formats, and biosimilar activity are simultaneously creating new opportunities and elevating execution risks across manufacturing, market access, and provider adoption. Regulatory and policy developments, including anticipated trade adjustments, further underscore the need for agile sourcing strategies and focused investments in manufacturing and distribution resilience.Consequently, organizations that proactively align evidence generation with payer expectations, invest in differentiated delivery and service models, and shore up supply chain flexibility will be best positioned to sustain access and capture long-term value. The cumulative picture is one of dynamic evolution rather than abrupt disruption, offering room for strategic initiatives that balance near-term operational continuity with deliberate, evidence-driven growth programs.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Biologic Pegfilgrastim
- Biosimilars
- Dosage Type
- Pre-filled Syringes
- Vials
- Route of Administration
- Intravenous Injection
- Intravitreal Injection
- Application
- Diabetic Macular Edema (DME)
- Diabetic Retinopathy (DR)
- Myopic Choroidal Neovascularization (mCNV)
- Retinal Vein Occlusion (RVO)
- Wet Age-related Macular Degeneration (wAMD)
- End User
- Ambulatory Surgical Centers (ASCs)
- Hospitals
- Ophthalmology Centers
- Distribution Channel
- Offline
- Online
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Apotex Inc.
- Bayer Aktiengesellschaft
- Biocon Limited
- Celltrion, Inc.
- Formycon AG
- Fresenius Kabi AG
- Gene Techno Science Co., Ltd.
- Intas Pharmaceuticals Limited
- Mylan N.V.
- Novartis AG
- Qilu Pharmaceutical Co., Ltd.
- Regeneron Pharmaceuticals, Inc.
- Samsung Bioepis Co., Ltd.
- Sartorius AG
- STADA Arzneimittel AG
- Teva Pharmaceutical Industries Ltd.
Table of Contents
20. ResearchStatistics
21. ResearchContacts
22. ResearchArticles
23. Appendix
Companies Mentioned
The companies profiled in this Aflibercept market report include:- Apotex Inc.
- Bayer Aktiengesellschaft
- Biocon Limited
- Celltrion, Inc.
- Formycon AG
- Fresenius Kabi AG
- Gene Techno Science Co., Ltd.
- Intas Pharmaceuticals Limited
- Mylan N.V.
- Novartis AG
- Qilu Pharmaceutical Co., Ltd.
- Regeneron Pharmaceuticals, Inc.
- Samsung Bioepis Co., Ltd.
- Sartorius AG
- STADA Arzneimittel AG
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
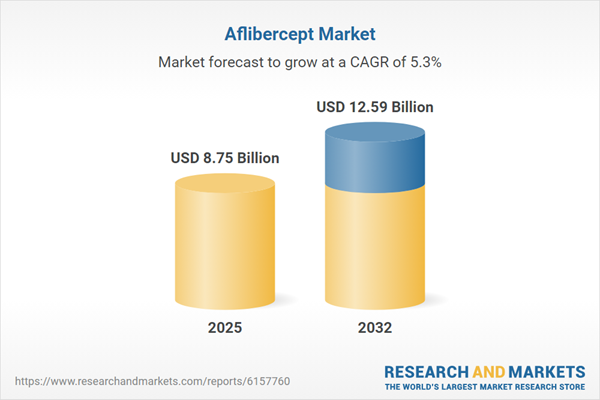
| Estimated Market Value ( USD | $ 8.75 Billion |
| Forecasted Market Value ( USD | $ 12.59 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 17 |