Speak directly to the analyst to clarify any post sales queries you may have.
A strategic introduction outlining how quality assurance, regulatory alignment, and laboratory capacity are redefining competitive advantage in juice testing
The juice testing sector sits at the intersection of food safety, consumer expectations, and evolving regulatory regimes. Rapid changes in supply chain complexity and consumer demand for transparency have pushed quality assurance from a back-office compliance function to a strategic differentiator. In this environment, laboratory testing capabilities and testing program design are no longer purely technical issues; they are core components of brand trust and market access strategies. Consequently, decision-makers must reconcile operational realities such as sample throughput, turnaround times, and cost per analysis with broader concerns including label accuracy, adulteration risks, and claims verification.To address these demands, stakeholders are investing in both in-house laboratory capacity and outsourced partnerships, while also refining testing protocols to match product innovation such as cold-pressed formats and novel blends. This introduction frames the immediate priorities for manufacturers, regulators, and laboratory service providers: align testing approaches with product complexity, prioritize high-impact analytes and methods, and integrate testing outcomes into commercial and regulatory decision-making processes. A focused approach will reduce downstream risk, expedite market introductions, and preserve consumer confidence in an increasingly scrutinized category.
An analysis of converging technological, regulatory, and consumer-driven forces that are fundamentally reshaping quality verification practices across the juice sector
Over the past several years the landscape for juice testing has been transformed by a convergence of technological progress, regulatory tightening, and shifting consumer expectations. Rapid advancements in analytical instrumentation have expanded the range, sensitivity, and throughput of tests that are practicable for routine quality control. Parallel to this, regulators in multiple jurisdictions have clarified requirements around authenticity, contaminants, and labeling claims, prompting processors to adopt more robust testing regimens and documented controls.Consumer trends toward clean labels, functional ingredients, and niche formats such as cold-pressed and blended juices have increased the diversity of analytes of interest, from botanical markers to nutrient profiles, and have required laboratories to adapt method portfolios. At the same time, supply chain globalization has intensified the need for harmonized standards and cross-border testing compatibility. Taken together, these transformative shifts place a premium on flexible testing strategies that incorporate both classical methods and emergent technologies, and on data systems that translate analytical results into operational and compliance outcomes.
How recent tariff shifts through 2025 have altered sourcing, supply chain risk, and the demand for enhanced authenticity and contaminant testing across juice supply chains
The cumulative effects of tariff adjustments and trade policy changes enacted through 2025 have created material operational considerations for stakeholders across the juice value chain. Tariff-induced cost pressures have prompted some suppliers and processors to re-evaluate sourcing strategies, with a resulting ripple effect on ingredient provenance and the need for intensified authenticity and adulteration testing. Where suppliers have shifted to alternate origins, laboratories and quality teams must adapt reference libraries and validation datasets to reflect new raw material matrices and potential adulterants.In addition, tariff-driven shifts in sourcing can shorten or complicate supply chains, increasing the likelihood of variability in raw input quality and placing greater emphasis on incoming goods inspection and contaminant testing. Regulatory compliance teams face the dual task of monitoring trade policy impacts while ensuring that altered supply relationships do not create gaps in documentation or testing coverage. As a consequence, organizations are prioritizing dynamic sampling plans and strengthening contractual testing obligations with suppliers to preserve market access and manage reputational risk in a landscape where trade policy and food safety are increasingly intertwined.
Comprehensive segmentation-driven insights mapping how test types, analytical technologies, product formats, applications, and end-user demands shape prioritized testing strategies
A nuanced segmentation perspective clarifies where testing efforts and investments will be most effective across product types, methodologies, applications, and end-user needs. Testing by type spans authenticity and adulteration testing, chemical testing, contaminant testing, microbiological testing, and nutritional testing, each of which addresses distinct risk profiles and claim verification requirements. Methodological segmentation highlights that chromatography methods including gas chromatography and high-performance liquid chromatography, polymerase chain reaction techniques in both conventional and real-time formats, and spectroscopy approaches such as near-infrared and ultraviolet-visible spectroscopy form the backbone of contemporary analytical portfolios, with each technology offering unique balances of sensitivity, throughput, and cost.Product segmentation demonstrates divergent testing imperatives among blended juices, cold-pressed formats, concentrated juices, fruit juices and vegetable juices; within fruit juices, apple, berry, citrus, pomegranate and tropical variants present differing adulteration and quality markers, while vegetable juices such as beetroot, carrot, spinach and tomato require tailored contaminant and nutrient assays. Application-driven testing ranges from certification and labeling support to product development, quality control, regulatory compliance and shelf-life and stability testing, shaping laboratory priorities and method validation needs. End-user segmentation encompasses food and beverage processing companies, regulatory and government agencies, research and academic institutions, and retailers and supermarkets, each demanding distinct reporting formats, turnaround expectations, and evidentiary standards for decisions related to market entry, certification, and consumer assurance.
Regional intelligence explaining how divergent regulatory environments, supply chain geographies, and consumer preferences are driving differentiated testing priorities globally
Regional dynamics materially influence testing priorities and resource allocation as stakeholders navigate divergent regulatory regimes, supply chain geographies, and consumer preferences. In the Americas, emphasis remains on large-scale processing operations, traceability for export markets, and robust contaminant screening; investment in high-throughput chromatography and PCR capabilities supports volume-driven quality control and regulatory compliance needs. Meanwhile, Europe, Middle East & Africa features a complex tapestry of standards where harmonization efforts, stringent labeling laws, and heightened focus on authenticity are driving demand for specialized analytical methods and validated reference materials.Asia-Pacific exhibits rapid product innovation and a broad spectrum of manufacturing sophistication, with particular demand for adaptable testing platforms that can support both modern cold-pressed niche producers and high-volume concentrated juice lines. Across regions, local regulatory interpretations, infrastructure maturity, and prevailing consumer trust issues determine the balance between in-house testing and third-party laboratory use. Consequently, market actors must align technical investments and compliance strategies to regional realities while maintaining the flexibility to respond to cross-border trade and evolving standards.
An evaluation of competitive positioning, specialization trends, and partnership models defining service providers, technology vendors, and in-house laboratory strategies
Competitive dynamics in the juice testing ecosystem are defined by specialization, vertical integration, and strategic partnerships rather than by uniform scale advantages alone. A spectrum of laboratory service providers competes on analytical breadth, method validation expertise, and the ability to support regulatory submissions and certification programs. Some entities focus on high-sensitivity contaminant and authenticity assays, investing in method development and reference library expansion, while others emphasize rapid, cost-effective screening approaches to support routine quality control in high-volume processing environments.Processors and retailers that internalize testing capabilities often do so to improve control over turnaround times and data ownership, while those that prioritize flexibility rely on external laboratories to access specialized instrumentation and cross-matrix experience. Alongside service providers, technology vendors offering chromatography, PCR, and spectroscopic instrumentation are positioning their value through workflow integration, software-enabled data analytics, and consumables ecosystems that reduce total cost of ownership. Strategic alliances between laboratories, instrument manufacturers, and certification bodies are emerging as a practical route to deliver end-to-end solutions that address both technical and compliance needs.
Actionable strategic priorities for manufacturers, laboratories, and retailers to enhance testing rigor, supplier assurance, and data-driven product governance
Industry leaders should prioritize a set of pragmatic actions to strengthen testing programs and reduce commercial and regulatory risk. First, align testing portfolios with product complexity by mapping critical analytes across juice types and adapting methods such as chromatography, PCR, and spectroscopy to capture authenticity, contaminant, and nutrient profiles in a cost-effective manner. Second, invest in validated method transfers and inter-laboratory comparability to ensure that results from alternate suppliers or contract laboratories are defensible for regulatory and commercial dispute resolution.Third, strengthen supplier assurance through tighter contractual testing requirements and conditional acceptance criteria, particularly when sourcing changes in response to trade or tariff pressures. Fourth, integrate testing outputs into governance processes so that analytic findings drive product release decisions, labeling claims, and shelf-life management; transparency in data and traceability will support both risk mitigation and consumer trust. Finally, evaluate hybrid approaches that combine in-house rapid screening with external confirmatory analysis to balance speed, cost, and depth of insight. Executing these measures will improve resilience and support sustainable growth across diverse market and regulatory contexts.
A transparent research methodology detailing triangulated analyses from regulatory review, technical literature synthesis, and practitioner interviews to underpin practical recommendations
The research approach underpinning this analysis combined a structured review of contemporary regulatory texts, a technical synthesis of peer-reviewed analytical method literature, and qualitative interviews with laboratory directors, quality assurance leads, and regulatory specialists. Data triangulation emphasized methodological validation practices and operational constraints such as throughput requirements, sample matrix effects, and cost-per-test considerations. Where applicable, standard laboratory accreditation frameworks and proficiency testing outcomes were used to gauge methodological maturity and inter-laboratory reliability.Analytical conclusions were drawn by mapping test types to product categories and regional regulatory contexts, and by assessing technology suitability for routine quality control versus specialized forensic analyses. Confidential industry interviews provided practitioner perspectives on implementation barriers, while cross-referencing of published method protocols ensured that recommendations reflect accepted scientific practice. Throughout, emphasis was placed on transparency of methodological choices and the replicability of recommended workflows in commercial laboratory settings.
A concise conclusion stressing the need for layered testing programs, validated protocols, and integrated data governance to secure compliance and consumer trust
Bringing together technological capability, regulatory attention, and the commercial imperative for brand trust yields a clear imperative: testing strategy must be proactive, adaptable, and closely linked to business objectives. Analytical diversity is necessary because no single method can address the full spectrum of authenticity, contaminant, microbiological, and nutritional questions that arise across juice formats. Consequently, laboratories and quality teams should design layered testing programs that combine rapid screening with confirmatory assays and maintain validated protocols for the product and matrix in question.At the organizational level, embedding testing outcomes into product development cycles, supplier qualification processes, and labeling governance will reduce time-to-market friction and minimize recall risk. The conclusion is that disciplined investment in method capability, data governance, and supplier controls delivers both risk reduction and competitive value by enabling credible claims and consistent product quality in a market where consumers and regulators are increasingly vigilant.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Test Type
- Authenticity & Adulteration Testing
- Chemical Testing
- Contaminant Testing
- Microbiological Testing
- Nutritional Testing
- Technology
- Chromatography
- Gas Chromatography (GC)
- High-Performance Liquid Chromatography (HPLC)
- PCR
- Conventional PCR
- Real-time PCR
- Spectroscopy
- NIR Spectroscopy
- Ultraviolet-Visible Spectroscopy
- Chromatography
- Juice Type
- Blended Juice
- Cold-Pressed Juice
- Concentrated Juice
- Fruit Juice
- Apple
- Berry
- Citrus
- Pomegranate
- Tropical
- Vegetable Juice
- Beetroot
- Carrot
- Spinach
- Tomato
- Application
- Certification & Labeling
- Product Development
- Quality Control
- Regulatory Compliance
- Shelf-life & Stability Testing
- End-User
- Food and Beverage Processing Companies
- Regulatory & Government Agencies
- Research & Academic Institutions
- Retailers & Supermarkets
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- ALS Limited
- Bureau Veritas SA
- Eurofins Scientific SE
- Intertek Group plc
- Mérieux NutriSciences Holding SAS
- NSF International
- Q Laboratories, Inc.
- SGS SA
- TÜV SÜD GmbH
- Underwriters Laboratories LLC
- Certified Laboratories, Inc.
- Symbio Laboratories Pty Ltd
- Alfa Chemistry LLC
- FoodChain ID, Inc.
- AGQ Labs USA, Inc.
- Tentamus Group GmbH
- Lifeasible, Inc.
- Pacific Laboratory Products Pty Ltd
- LifeAnalytics Corporation
- Pony Testing International Group Co., Ltd.
- China Certification & Inspection (Group) Co., Ltd.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Juice Testing market report include:- ALS Limited
- Bureau Veritas SA
- Eurofins Scientific SE
- Intertek Group plc
- Mérieux NutriSciences Holding SAS
- NSF International
- Q Laboratories, Inc.
- SGS SA
- TÜV SÜD GmbH
- Underwriters Laboratories LLC
- Certified Laboratories, Inc.
- Symbio Laboratories Pty Ltd
- Alfa Chemistry LLC
- FoodChain ID, Inc.
- AGQ Labs USA, Inc.
- Tentamus Group GmbH
- Lifeasible, Inc.
- Pacific Laboratory Products Pty Ltd
- LifeAnalytics Corporation
- Pony Testing International Group Co., Ltd.
- China Certification & Inspection (Group) Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
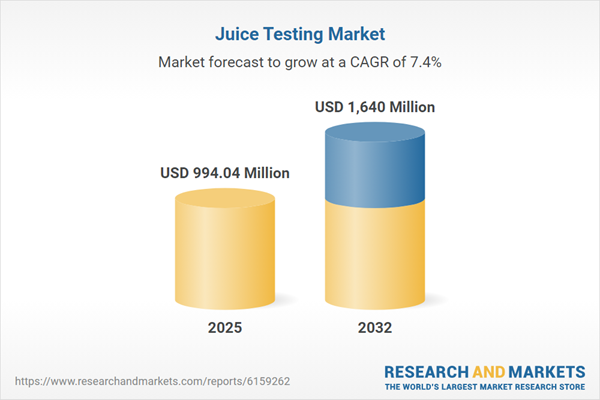
| Estimated Market Value ( USD | $ 994.04 Million |
| Forecasted Market Value ( USD | $ 1640 Million |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |