Speak directly to the analyst to clarify any post sales queries you may have.
Unveiling the Transformative Market Drivers and Strategic Imperatives Defining Single Use Surgical Instruments Adoption
The single use surgical instruments market continues to capture the attention of healthcare leaders seeking to enhance patient safety and operational efficiency. Driven by a convergence of clinical priorities, regulatory imperatives, and technological innovation, the shift toward disposables has accelerated across a broad spectrum of surgical specialties. Infection control remains paramount, and single use instruments have emerged as a critical component in reducing cross-contamination risks while streamlining instrument management protocols.In parallel, advancements in materials science and manufacturing techniques have bolstered the performance and reliability of disposable tools, fostering confidence among surgeons and procurement professionals alike. Moreover, economic pressures to optimize inventory costs and minimize sterilization overhead are reshaping procurement strategies. Against this backdrop, a nuanced understanding of market dynamics-from supply chain constraints to regional variances in healthcare infrastructure-has become indispensable for stakeholders aiming to secure a competitive edge.
This introduction sets the stage for a comprehensive exploration of how transformative shifts, trade policy changes, and segmentation insights are redefining the single use surgical instruments landscape. By examining these core elements in depth, readers will gain a clearer perspective on where opportunities lie and how to anticipate future market developments.
Identifying the Pivotal Technological Innovations and Sustainability Imperatives Reshaping Disposable Surgical Instruments
The single use surgical instruments space is undergoing profound transformation fueled by technological innovation and evolving clinical needs. Cutting-edge manufacturing techniques such as micro-injection molding and additive manufacturing have unlocked new possibilities in precision, ergonomic design, and cost-effective production. Consequently, surgical teams are benefiting from instruments that not only meet stringent regulatory requirements but also deliver improved functionality and tactile feedback akin to reusable counterparts.Simultaneously, growing emphasis on environmental sustainability is prompting manufacturers to explore eco-friendly materials and end-of-life disposal solutions. These initiatives reflect a broader industry shift toward balancing high performance with responsible resource stewardship. At the same time, regulatory bodies worldwide are refining guidelines to ensure product safety, quality, and traceability, further shaping product development pipelines.
Amidst these changes, strategic partnerships and research collaborations between healthcare institutions, material scientists, and device manufacturers have become increasingly common. This collaborative ecosystem accelerates the translation of novel concepts into market-ready solutions, setting a new benchmark for patient care. By navigating these transformative currents, industry participants are poised to capture value and lead the next wave of innovation in disposable surgical instrumentation.
Examining the Comprehensive Impact of 2025 United States Tariff Adjustments on Disposable Surgical Instruments Supply Chains
Beginning in early 2025, the implementation of revised tariff schedules by United States authorities has introduced new cost dynamics for imported single use surgical instruments and their constituent materials. Instruments previously subject to minimal duties now face incremental levies, particularly those containing stainless steel and specialty polymers. These measures were announced with the intent to bolster domestic manufacturing, but they also carry implications for cost structures and procurement strategies across the healthcare ecosystem.As a result, many multinational suppliers have reevaluated their sourcing and manufacturing footprints, accelerating initiatives to localize production and qualify alternative material suppliers. This transition has required close collaboration with contract manufacturers and rigorous validation protocols to maintain quality and regulatory compliance. Simultaneously, healthcare providers are recalibrating their vendor agreements to reflect the new cost paradigm, often negotiating volume-based contracts or exploring group purchasing arrangements to offset tariff-induced price adjustments.
Over time, these shifts are expected to yield a more resilient supply chain, albeit one that demands greater agility in responding to policy changes. By understanding the cumulative impact of these tariffs, market participants can anticipate cost pressures, identify strategic sourcing alternatives, and position themselves to capitalize on emerging domestic manufacturing incentives.
Navigating the Interplay of Instrument Type Material Application End User and Distribution Dynamics in Market Segmentation
A nuanced analysis of market segmentation reveals that instrument type, material composition, clinical application, end user, and channel dynamics each play a critical role in shaping competitive landscapes. Among instrument type categories, electrosurgical instruments continue to benefit from ongoing enhancements in energy delivery systems, while handheld instruments-encompassing forceps, needle holders, retractors, and scissors-remain fundamental to general surgery procedures. In parallel, specialized instruments such as clip appliers, dilators, speculums, staplers, suction tubes, and trocar cannulas are gaining traction in minimally invasive and specialized surgical suites.Material selection further differentiates product offerings. Aluminum alloys are prized for their lightweight durability, composites bring advanced performance characteristics, plastics deliver cost efficiency and sterilization ease, and stainless steel upholds rigorous strength and corrosion resistance standards. The choice of material often aligns with clinical requirements and cost considerations, influencing adoption rates across healthcare settings.
Application segmentation underscores diverse usage patterns, from cardiovascular surgery and ENT procedures to gynecology, neurosurgery, ophthalmology, orthopedic surgery, and urology. Each specialty introduces unique instrument design specifications and regulatory prerequisites. End users range from ambulatory surgical centers, which prioritize rapid turnover and cost containment, to hospitals and clinics demanding broad product portfolios and rigorous quality assurance processes. Distribution channels oscillate between direct sales models offering tailored service packages and distributor networks that deliver wide geographic reach and logistical support.
Exploring Unique Regional Dynamics and Growth Catalysts Across Americas Europe Middle East Africa and Asia Pacific Markets
Regional dynamics in the single use surgical instruments market reflect distinct healthcare infrastructure maturity levels, regulatory frameworks, and economic imperatives. In the Americas, robust investment in advanced surgical technologies and stringent infection control protocols continue to drive disposable instrument adoption. Providers in North America benefit from integrated supply chain models and reimbursement structures that recognize the long-term cost savings associated with minimized sterilization and reprocessing overhead.Europe Middle East & Africa (EMEA) presents a diverse tapestry of market conditions. Western European markets exhibit high standards for device traceability and environmental impact reduction, prompting manufacturers to emphasize sustainable design and recycling programs. Meanwhile, emerging markets in the Middle East and parts of Africa are characterized by rapid healthcare facility expansion and demand for cost-effective solutions, offering fertile ground for targeted product innovations.
In Asia-Pacific, accelerating surgical volumes, growing medical tourism hubs, and expanding consciousness of patient safety are fueling demand for reliable disposable instruments. Governments across the region are increasingly supporting domestic production through favorable policies, further intensifying competition. Across all regions, strategic partnerships with local distributors, adaptability to regional regulatory protocols, and investments in value-added services remain critical to capturing and sustaining market share.
Profiling the Strategic Initiatives and Competitive Dynamics Shaping Leading Disposable Surgical Instruments Manufacturers
Leading manufacturers in the single use surgical instruments arena are deploying a blend of strategic initiatives to consolidate market position and spur innovation. Product portfolio expansion remains a cornerstone, with companies introducing next-generation electrosurgical tools, ergonomic handheld sets, and specialized instrument lines tailored to emerging minimally invasive techniques. These offerings are often supported by digital integration features, such as instrument usage tracking systems designed to optimize OR efficiency and compliance.Mergers, acquisitions, and strategic alliances have reshaped the competitive landscape, enabling rapid entry into adjacent market segments and augmenting global manufacturing capabilities. In addition, several market participants are forging research partnerships with academic institutions and healthcare networks to co-develop prototypes and clinical validation programs. Sustainability efforts are also gaining prominence, as leading firms explore biodegradable polymers and take-back programs to address environmental concerns.
Taken together, these strategic maneuvers underscore a collective emphasis on differentiation through performance, regulatory adherence, and service excellence. As industry competition intensifies, the ability to anticipate surgical practice shifts and respond with targeted offerings will define the next wave of market leadership.
Actionable Strategic Recommendations to Enhance Innovation Agility Supply Chain Resilience and Market Penetration
Industry leaders seeking to fortify their market presence should prioritize investments in research and development focused on advanced materials and smart instrument integration. By fostering collaborations with key opinion leaders and clinical partners early in the design process, manufacturers can ensure that new products address real-world surgical challenges and gain faster market acceptance.Furthermore, establishing localized production capabilities or flexible manufacturing partnerships can mitigate the impact of trade policy fluctuations and enhance supply chain resilience. Aligning these operational strategies with robust quality management systems and rapid regulatory approval pathways will accelerate time-to-market and reduce risk exposure.
From a commercial standpoint, tailoring channel strategies to match regional preferences-leveraging direct sales for high-complexity products and distributor networks for broader market coverage-will optimize go-to-market efficiency. Complementing product launches with value-added services such as training programs, digital analytics platforms, and instrument lifecycle management solutions will further differentiate offerings and strengthen customer loyalty.
By executing these recommendations with agility and foresight, industry participants can navigate evolving clinical, regulatory, and economic landscapes, unlocking sustainable growth in the competitive disposable surgical instruments sector.
Detailing the Rigorous Multi Stage Qualitative and Quantitative Research Framework Underpinning Market Insights
This market research study employs a comprehensive, multi-stage methodology integrating both qualitative and quantitative analyses. Primary research involved in-depth interviews with key opinion leaders including surgeons, procurement managers, and industry executives to capture firsthand insights on clinical adoption trends and purchasing criteria. Secondary research encompassed a thorough review of published literature, regulatory filings, technical whitepapers, and industry association reports to corroborate market dynamics and regulatory frameworks.Quantitative data points were triangulated through multiple sources, ensuring robustness and validity. Market segmentation hypotheses were validated by cross-referencing product portfolios of leading manufacturers, regional trade databases, and healthcare utilization statistics. Regional market assessments incorporated macroeconomic indicators, healthcare infrastructure indices, and policy developments to contextualize growth drivers and potential barriers.
Analytical frameworks such as SWOT analysis, Porter's Five Forces, and value chain mapping were applied to evaluate competitive intensity, supplier-buyer relationships, and innovation pipelines. Risk factors including tariff impacts, regulatory shifts, and sustainability imperatives were systematically examined to inform scenario planning. Throughout the process, data quality checks and peer reviews were conducted to uphold methodological rigor and ensure actionable insights for decision-makers.
Synthesizing Key Insights and Strategic Imperatives Guiding Stakeholders in the Disposable Surgical Instruments Arena
The evolving single use surgical instruments market is characterized by a confluence of technological advancement, regulatory evolution, and shifting clinical priorities. From the integration of smart materials and sustainable manufacturing practices to the strategic responses necessitated by new tariff regimes, stakeholders must continuously adapt to maintain competitive advantage. The detailed segmentation analysis underscores the importance of tailoring offerings to specific instrument types, material preferences, clinical applications, end users, and distribution channels.Regional insights reveal that market potential varies significantly across the Americas, Europe Middle East & Africa, and Asia-Pacific, each presenting unique opportunities and challenges. Leading companies are responding through diversified product portfolios, strategic collaborations, and targeted investments in localized production and regulatory compliance initiatives.
Looking ahead, success in this market will hinge on the ability to anticipate surgeon requirements, streamline supply chain operations, and proactively address sustainability concerns. By synthesizing the key findings outlined in this report, organizations can chart a strategic course that balances innovation with operational resilience, ensuring they remain at the forefront of disposable surgical instrument development and delivery.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Instrument Type
- Electrosurgical Instruments
- Handheld Instruments
- Forceps
- Needle Holders
- Retractors
- Scissors
- Specialized Instruments
- Clip Appliers
- Dilators
- Speculums
- Staplers
- Suction Tubes
- Trocar Cannulas
- Material Type
- Aluminum Alloys
- Composite
- Plastic
- Stainless Steel
- Application
- Cardiovascular Surgery
- ENT
- General Surgery
- Gynecology
- Neurosurgery
- Ophthalmology
- Orthopedic Surgery
- Urology
- End User
- Ambulatory Surgical Centers
- Hospitals & Clinics
- Distribution Channel
- Direct Sales
- Distributors
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Acumed LLC
- Alcon Laboratories, Inc
- Apothecaries Sundries Mfg. Pvt. Ltd.
- Atlas Surgical
- B. Braun SE
- Bhatt Surgicals
- BOSS Instruments, Ltd
- CooperSurgical, Inc
- DTR Medical Ltd
- Kapp Surgical Instrument Inc.
- Medisafe International
- Medtronic PLC
- Mercator Medical S.A.
- Paragon Medical
- Sklar Surgical Instruments
- Smith+Nephew PLC
- Steris plc
- Stryker Corporation
- Surgical Holdings Ltd
- Swann-Morton Ltd
- Victor Medical Instruments Co., Ltd.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Single Use Surgical Instruments market report include:- Acumed LLC
- Alcon Laboratories, Inc
- Apothecaries Sundries Mfg. Pvt. Ltd.
- Atlas Surgical
- B. Braun SE
- Bhatt Surgicals
- BOSS Instruments, Ltd
- CooperSurgical, Inc
- DTR Medical Ltd
- Kapp Surgical Instrument Inc.
- Medisafe International
- Medtronic PLC
- Mercator Medical S.A.
- Paragon Medical
- Sklar Surgical Instruments
- Smith+Nephew PLC
- Steris plc
- Stryker Corporation
- Surgical Holdings Ltd
- Swann-Morton Ltd
- Victor Medical Instruments Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | November 2025 |
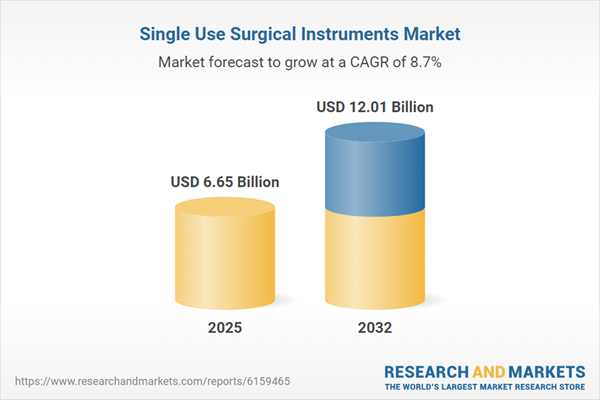
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 6.65 Billion |
| Forecasted Market Value ( USD | $ 12.01 Billion |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |