Speak directly to the analyst to clarify any post sales queries you may have.
A concise overview of how evolving clinical practices, caregiver awareness, and technology have collectively redefined pet diabetes care delivery and expectations
Pet diabetes care has evolved from a sporadically addressed veterinary niche to a sustained, multidisciplinary area of clinical practice, product development, and owner-driven care strategies. Rising pet longevity, heightened awareness of chronic disease management among caregivers, and improved diagnostic accessibility have collectively reshaped how clinicians and owners identify and manage diabetes in companion animals. In turn, this has created a complex ecosystem of medical devices, nutraceutical approaches, and pharmaceutical therapies that interact with everyday pet care routines.Moving from recognition to routine management has required improvements in clinical guidelines, more robust owner education, and cross-functional coordination between general practice veterinarians, specialists, and suppliers. These changes underpin a renewed focus on adherence, humane delivery methods, and technologies that minimize stress for animals and their caregivers. As a result, stakeholders are increasingly prioritizing interventions that balance clinical efficacy with quality-of-life considerations, while also recognizing the economic and logistical realities of long-term disease control.
How technological miniaturization, digital care models, and owner-centric innovations are rapidly reshaping long-term diabetes management for companion animals
The pet diabetes landscape is undergoing transformative shifts driven by technological convergence, behavioral science insights, and changes in how care is delivered across home and clinical settings. Continuous glucose monitoring concepts adapted from human medicine are being miniaturized and calibrated for companion animals, enabling more frequent and less invasive glycemic profiling. At the same time, innovations in insulin formulations and delivery systems aim to reduce dosing complexity and improve stability under typical household conditions.Concurrently, digital platforms that enhance owner education and remote clinical oversight have gained traction, allowing veterinary professionals to monitor adherence and outcomes between visits. This shift toward integrated care models has also prompted a reevaluation of product portfolios, with manufacturers seeking to pair therapeutics with services and support solutions that address both clinical endpoints and owner experience. Together, these forces are accelerating the transition from episodic interventions to continuous, data-informed management strategies that emphasize prevention of complications and preservation of animal welfare.
Assessing how recent tariff shifts in the United States have reconfigured supply chains, procurement tactics, and operational resilience across the pet diabetes ecosystem
Recent tariff developments in the United States have introduced additional complexity to supply chains and procurement strategies for pet diabetes-related products. Increased import duties on medical devices and certain specialty nutritional items have affected costs downstream, prompting manufacturers and distributors to reassess source regions, packaging configurations, and inventory strategies. As a result, some stakeholders have shifted toward regional manufacturing or diversified supplier networks to mitigate exposure to sudden policy changes.These adjustments have implications beyond pricing: they influence lead times, regulatory paperwork, and the selection of materials compatible with local manufacturing capabilities. Veterinary clinics and large retail distributors have responded by tightening procurement windows, prioritizing vendors with nearshore capabilities, and increasing emphasis on predictable service levels. Meanwhile, smaller independent practices and specialty retailers face additional friction as they balance the need for clinically appropriate products with sensitivity to price changes and stock variability. Ultimately, policy shifts have catalyzed operational innovation, accelerating adoption of resilient sourcing and inventory techniques throughout the value chain.
Integrated segmentation perspectives that illuminate how product categories, species differences, disease phenotypes, user roles, and distribution channels uniquely influence care pathways and commercialization
Segment-level insights reveal distinct dynamics across product, pet, disease, end-user, and channel categories that shape how care is delivered and solutions are commercialized. Considering product type, diabetic pet food has become central to nutritional management strategies that complement pharmacologic care, while glucose monitoring devices increasingly enable home-based decision support and insulin therapies remain the core clinical intervention for many patients. For pet type, dogs often present with different epidemiology and owner care patterns than cats, which influences device ergonomics, dosing regimens, and educational needs tailored to each species.Examining disease type clarifies that type 1 diabetes requires different monitoring and therapeutic emphases compared with type 2 diabetes, where lifestyle and nutritional interventions can play a larger role. In terms of end user, pet owners demand intuitive and minimally disruptive products and clear guidance, whereas veterinary professionals prioritize clinical reliability, data fidelity, and integration with practice workflow. Finally, distribution channel distinctions between offline and online environments affect purchasing behavior, return policies, and the availability of professional support, with online channels expanding access but offline channels retaining importance for hands-on training and immediate clinical consultation.
A nuanced regional assessment of how distinct regulatory environments, consumer behaviors, and infrastructure priorities shape product uptake and clinical adoption globally
Regional nuances have substantial influence on regulatory pathways, product availability, and clinical practices across the globe. In the Americas, a mature veterinary services market coupled with strong consumer spending power has accelerated adoption of advanced diagnostics and monitoring tools, while also emphasizing value propositions tied to quality-of-life improvements and convenience for caregivers. This region frequently spearheads clinical studies and early commercial introductions that shape global standards.In Europe, Middle East & Africa, regulatory heterogeneity and variable access to veterinary services create a mosaic of opportunities and constraints; some countries exhibit high uptake of specialist care and digital health platforms, whereas others rely more heavily on nutritional strategies and primary care interventions. Asia-Pacific demonstrates rapid growth in companion animal ownership and investment in veterinary infrastructure, with urban markets showing fast adoption of premium products and telemedicine models. These regional trends collectively drive cross-border partnerships, with each geography contributing distinct regulatory insights, consumer expectations, and distribution norms that influence global strategy.
Strategic corporate behaviors and alliance models that drive competitive differentiation through product usability, clinician engagement, and integrated care solutions
Key company strategies in the pet diabetes space center on product differentiation, service integration, and collaborations that extend clinical reach. Manufacturers that prioritize ease-of-use, animal comfort, and interoperability with digital platforms typically secure stronger relationships with veterinary professionals and caregivers. At the same time, firms that invest in educational programs, outcome-tracking tools, and clinician training establish credibility that supports sustained product adoption and loyalty.Strategic partnerships between device makers, pharmaceutical suppliers, and pet nutrition companies are emerging to offer bundled solutions that address the multifactorial needs of diabetic animals. Additionally, companies that develop robust post-market support and data analytics capabilities gain competitive advantages by delivering real-world evidence that informs iterative product improvements. Across the value chain, agility in responding to supply chain disruptions, regulatory changes, and evolving clinical protocols remains a critical differentiator for sustained market relevance.
Practical and prioritized actions for companies to strengthen clinical integration, supply chain resilience, and caregiver-centered product adoption across the pet diabetes continuum
Industry leaders should adopt an integrated approach that aligns product development with clinical workflows and caregiver realities. Prioritize designing devices and therapies that reduce complexity for owners and improve tolerability for pets, while ensuring that veterinary professionals can incorporate new tools without disrupting practice efficiency. Invest in clinician education and certification programs that translate product features into practical treatment protocols, and couple these initiatives with outcome-tracking to demonstrate real-world benefit.Operationally, companies should diversify sourcing strategies and build nearshore capabilities where feasible to mitigate tariff-related volatility and shorten replenishment cycles. Expand channel strategies by strengthening partnerships with both brick-and-mortar clinics and digital distributors, and create omnichannel experiences that provide hands-on onboarding supplemented by remote support. Finally, prioritize collaborative research with veterinary institutions and patient registries to generate robust clinical evidence that supports guideline adoption and informs iterative product enhancements.
A transparent description of the research approach that blends practitioner interviews, clinical literature review, and product intelligence synthesis to generate actionable insights
This analysis integrates a triangulated research methodology combining primary qualitative interviews with clinical specialists and industry executives, secondary review of peer-reviewed veterinary literature and regulatory guidance, and synthesis of product intelligence derived from publicly available technical specifications and clinical white papers. Primary research focused on eliciting practitioner perspectives on usability, adherence, and clinical outcomes, while secondary sources provided context on device design principles, therapeutic limitations, and regional regulatory frameworks.Data synthesis prioritized cross-validation across sources to ensure that emerging trends reflected both frontline clinical experience and documented product characteristics. Throughout the process, emphasis was placed on extracting actionable insights rather than quantitative projections, and on identifying operational levers that stakeholders can deploy to improve care delivery and commercial performance. The methodology aimed to balance depth of clinical understanding with pragmatic commercial intelligence to support decision-makers across stakeholders.
A forward-looking synthesis that highlights the essential interplay between clinical innovation, caregiver experience, and operational readiness necessary to advance pet diabetes care
In conclusion, pet diabetes care sits at the intersection of clinical rigor, caregiver behavior, and technological innovation. Evolving diagnostic tools, improved treatment modalities, and enhanced educational resources are converging to enable more consistent and humane management of diabetes in companion animals. These advances present opportunities for stakeholders to improve clinical outcomes while aligning products with the practical needs of pet owners and veterinary teams.However, success will require coordinated efforts across product design, clinician engagement, supply chain resilience, and evidence generation. By focusing on integrated solutions that simplify daily management, enhance data-driven decision-making, and anticipate regional regulatory variations, the industry can deliver meaningful improvements in animal welfare and caregiver experience. Continued collaboration among clinicians, manufacturers, and distributors will be essential to translate technological promise into measurable improvements in routine care.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Diabetic Pet Food
- Glucose Monitoring Devices
- Insulin Therapies
- Pet Type
- Cats
- Dogs
- Disease Type
- Type 1 Diabetes
- Type 2 Diabetes
- End User
- Pet Owners
- Veterinary Professionals
- Distribution Channel
- Offline
- Online
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- AccuBioTech Co., Ltd
- Allison Medical, Inc.
- ALR Technologies Inc
- Animal Diabetes Australia Pty Ltd
- Boehringer Ingelheim International GmbH
- Dechra Pharmaceuticals PLC
- Elanco Animal Health Incorporated
- Hill's Pet Nutrition, Inc.
- i-SENS, Inc.
- Lifecare ASA
- MED TRUST
- Merck & Co., Inc.
- Nova Biomedical
- Perrigo Company plc
- Taidoc Technology Corporation
- Trividia Health, Inc.
- UltiMed, Inc.
- Universal Biosensors Inc.
- Virbac S.A.
- Zoetis UK Limited
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Pet Diabetes Care market report include:- AccuBioTech Co., Ltd
- Allison Medical, Inc.
- ALR Technologies Inc
- Animal Diabetes Australia Pty Ltd
- Boehringer Ingelheim International GmbH
- Dechra Pharmaceuticals PLC
- Elanco Animal Health Incorporated
- Hill's Pet Nutrition, Inc.
- i-SENS, Inc.
- Lifecare ASA
- MED TRUST
- Merck & Co., Inc.
- Nova Biomedical
- Perrigo Company plc
- Taidoc Technology Corporation
- Trividia Health, Inc.
- UltiMed, Inc.
- Universal Biosensors Inc.
- Virbac S.A.
- Zoetis UK Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | November 2025 |
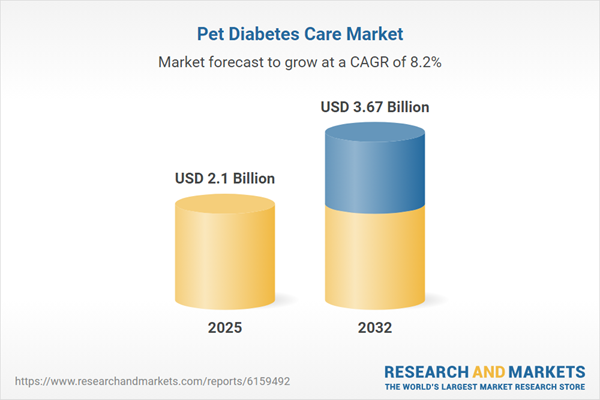
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 2.1 Billion |
| Forecasted Market Value ( USD | $ 3.67 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |