Speak directly to the analyst to clarify any post sales queries you may have.
Understanding the Emergence of Advanced Skin Diagnosis Systems Driven by Digital Transformation and Evolving Dermatological Needs Worldwide
The skin diagnosis landscape has undergone a profound transformation driven by both clinical necessity and technological innovation. As dermatological conditions continue to rise in prevalence across diverse patient populations, there is mounting pressure on healthcare providers to deliver more precise, rapid, and non-invasive diagnostic solutions. In response, developers have harnessed advances in imaging modalities, artificial intelligence algorithms, and sensor miniaturization to create systems that can detect subtle changes in skin health with unprecedented accuracy.This shift toward comprehensive digital platforms is marked by the convergence of non-invasive devices-capable of capturing high-resolution dermoscopic and ultrasound images-with sophisticated software solutions that leverage deep learning to interpret complex patterns. Concurrently, wearable sensors in the form of patches and wristbands are enabling continuous monitoring of skin physiology, opening new avenues for proactive care and remote patient management.
Collectively, these innovations are redefining the patient journey. From enhanced point-of-care assessments in dermatology clinics to home-based self-examinations using diagnostic apps, the landscape is moving toward greater accessibility and real-time insights. As the industry embraces interoperability and data portability, the fusion of hardware and software promises to elevate clinical decision-making, streamline treatment pathways, and ultimately improve patient outcomes.
Exploring the Pivotal Technological Shifts and Industry Collaborations Reshaping the Future of Non Invasive and AI-Driven Skin Assessment Solutions
The industry is witnessing a profound paradigm shift fueled by the rapid integration of artificial intelligence into diagnostic workflows. Deep learning models trained on large image repositories are now capable of distinguishing between benign and malignant lesions with accuracy levels approaching experienced clinicians. Alongside this, cloud-based platforms have enabled seamless collaboration between specialists and primary care providers, ushering in a new era of teledermatology.Equally transformative is the growing emphasis on personalized care through wearable sensors that track hydration, UV exposure, and even biochemical markers in sweat. These real-time data streams are informing dynamic treatment adjustments and fostering patient engagement by providing immediate feedback on therapeutic efficacy. Furthermore, strategic collaborations between device manufacturers, software developers, and academic research institutions are accelerating the commercialization of next-generation modalities.
Regulatory frameworks have also evolved to accommodate these cutting-edge solutions, with expedited pathways for AI-driven software and sensor-embedded devices. As convergence intensifies across hardware, software, and data analytics, the industry is positioned to deliver more holistic and proactive skin health management, transcending traditional episodic care models.
Assessing the Comprehensive Impact of United States Tariffs on Component Costs Supply Chains and Strategic Sourcing for Skin Diagnosis Technology in 2025
The introduction of elevated United States tariffs in 2025 has prompted a strategic reevaluation of global supply chains for critical components used in imaging, spectroscopy, and wearable sensors. Manufacturers that once depended heavily on imports of specialized optics and microelectronics have adopted a more diversified sourcing strategy, seeking regional suppliers to mitigate cost volatility. This shift has led to the development of localized assembly hubs and nearshore partnerships that enhance resilience against geopolitical disruptions.In parallel, R&D budgets have been reallocated to focus on component standardization and modular design architectures. By designing systems with interchangeable parts, companies can pivot more quickly when faced with sudden tariff adjustments or raw material scarcity. In some cases, proprietary software has been decoupled from hardware platforms, allowing end users to update diagnostic capabilities through cloud-based upgrades rather than purchasing entirely new devices.
While these adjustments have introduced short-term operational complexities, they have also spurred innovation in manufacturing processes and supply chain transparency. Companies that proactively engaged in scenario planning and forged alliances with regional distributors are now better positioned to deliver uninterrupted device maintenance and software support, ensuring sustained adoption of advanced skin diagnosis solutions.
Unveiling Critical Segmentation Insights Revealing Opportunities Across Device Categories Skin Types Applications User Profiles and Distribution Channels
An in-depth analysis of product type segmentation underscores the continued dominance of non-invasive devices, where imaging modalities such as dermoscopy and ultrasound imaging coexist with spectroscopy and thermal analysis to address diverse clinical requirements. Meanwhile, software solutions have evolved into sophisticated platforms that employ machine learning algorithms, while diagnostic apps deliver user-friendly interfaces for both clinicians and patients. Emerging wearable sensors, including adhesive patches and wristbands, are unlocking continuous monitoring capabilities that were once the purview of laboratory settings.When examining skin type segmentation, combination, dry, and oily skin categories each present unique diagnostic challenges and feature prominently in algorithm training sets. Simultaneously, applications ranging from acne diagnosis to lesion analysis, pigmentation mapping, psoriasis detection, and UV damage assessment are driving targeted innovation pipelines. Each application area demands tailored device specifications and data interpretation frameworks to ensure accuracy across diverse patient profiles.
End users span dermatology centers that rely on high-throughput imaging systems, home use scenarios powered by intuitive software apps, and hospitals and clinics seeking integrated diagnostic workstations. Distribution channels reflect a balance between direct sales for large institutional contracts, distributor networks that expand regional reach, and online sales platforms that cater to remote or individual practitioners. By understanding these interlocking segments, stakeholders can effectively align product development and go-to-market strategies with evolving customer needs.
Analyzing Regional Dynamics and Growth Drivers Shaping Distinct Market Adoption Patterns in the Americas EMEA and Asia-Pacific Territories
In the Americas, advanced skin diagnosis technologies have gained traction through partnerships with leading healthcare networks and supportive reimbursement models. The United States has emerged as a testbed for teledermatology pilots, while Canada's focus on rural access has driven investment in portable imaging and remote consultation platforms. Both markets exhibit strong demand for integrated solutions that streamline referral pathways and reduce time to diagnosis.Across Europe, the Middle East, and Africa, regulatory harmonization within the European Union has accelerated the approval of AI-driven software, while Middle Eastern health ministries focus on large-scale screening initiatives to address rising skin cancer incidence. Meanwhile, in parts of Africa, mobile clinics equipped with portable dermoscopic devices are bridging diagnostic gaps. This region's diversity in healthcare infrastructure has fostered a range of commercial models, from public-private screening programs to specialized urban clinics.
The Asia-Pacific region demonstrates rapid uptake in emerging economies, driven by growing awareness of skin health in urban populations and increasing digital literacy. Markets such as Japan and Australia lead in clinical validation studies, whereas Southeast Asian nations are embracing cost-effective wearables to expand access. Cross-border collaborations and government-backed innovation grants continue to fuel this region's dynamic growth trajectory.
Highlighting Strategic Innovations and Partnership Strategies Employed by Leading Corporations to Advance Skin Diagnosis Technologies and Market Leadership
Leading corporations have prioritized the integration of AI-driven analytics into their diagnostic portfolios, collaborating with research institutions to access curated image databases for model training. Some entrants have unveiled cloud-native platforms that support real-time lesion assessment and secure data sharing across distributed clinical teams. Others have focused on modular hardware architectures that allow end users to upgrade imaging modules without replacing entire devices.Strategic partnerships and targeted acquisitions have been a hallmark of the competitive environment. Device manufacturers have allied with software innovators to co-develop end-to-end solutions, while established digital health firms have acquired niche diagnostic app developers to bolster their product offerings. These alliances have accelerated time to market and broadened product roadmaps.
Mid-sized vendors and specialized startups continue to challenge incumbents by introducing disruptive sensor technologies and consumer-friendly diagnostic tools. As companies vie for leadership, intellectual property portfolios, regulatory approvals, and go-to-market agility have emerged as key differentiators. Those that effectively blend clinical-grade performance with ease of use are securing a growing share of both institutional and consumer segments.
Delivering Actionable Recommendations for Industry Leadership Through Collaboration Innovation and Regulatory Compliance in Skin Diagnosis Development
Industry leaders should prioritize the convergence of hardware and software through open platform architectures that facilitate third-party integrations and rapid feature rollouts. By adopting an API-driven approach, companies can extend their ecosystem and tap into specialized diagnostic apps developed by third parties. In addition, forging cross-industry alliances with telehealth providers and pharmacy chains can enhance distribution reach and patient engagement.To strengthen supply chain resilience, organizations must implement multi-sourcing strategies for critical components and invest in modular design principles that reduce dependency on proprietary parts. Concurrently, Agile methodologies applied to product development cycles can accelerate iteration and enable faster adaptation to regulatory changes or emerging clinical insights.
Finally, embracing user-centric design will be essential as the market expands beyond specialist settings into home use. Leaders should engage end users early through co-creation workshops and pilot programs to refine user interfaces and ensure that software-guided workflows align with real-world clinical routines. By championing these actionable priorities, industry participants can drive sustainable growth and deliver differentiated value.
Outlining Rigorous Research Methodologies Ensuring Data Integrity Transparency and Validated Insights in the Analysis of Skin Diagnosis System Technologies
This research was conducted through a structured multi-phase approach combining primary and secondary data sources. Primary research included interviews with key opinion leaders, dermatologists, device engineers, and regulatory experts to capture nuanced perspectives on unmet clinical needs, technology adoption barriers, and future innovation pathways. Market feedback sessions were held to validate preliminary findings and refine analytical frameworks.Secondary research encompassed a comprehensive review of scientific journals, peer-reviewed publications, patent filings, regulatory registrations, industry conference proceedings, and white papers from leading medical associations. Data points were triangulated across multiple sources to ensure consistency and reliability, with a focus on peer-reviewed evidence and documented clinical outcomes.
Quantitative analysis utilized thematic coding of interview transcripts and statistical correlation of adoption trends with macroeconomic indicators, while qualitative insights were distilled into strategic narratives that highlight best practices and emerging opportunities. The combined methodology ensures that the final deliverables offer both empirical rigor and actionable guidance.
Synthesizing Core Findings and Strategic Implications to Provide a Cohesive Outlook on the Future Trajectory of Skin Diagnosis Innovations
The evolving intersection of non-invasive diagnostic hardware, advanced analytics software, and wearable monitoring devices is redefining how skin health is assessed and managed. Key technological advancements, from enhanced imaging modalities to AI-driven interpretation engines, have improved diagnostic accuracy while expanding access through telehealth and home-based solutions. Meanwhile, the impact of geopolitical shifts such as tariff adjustments has underscored the importance of supply chain agility and modular design principles.Segmentation insights reveal that success hinges on aligning product offerings with specific end user needs-from high-capacity dermatology centers to individual consumers seeking convenient self-assessment tools. Regional dynamics further illustrate that tailored go-to-market strategies are essential, given the varying regulatory landscapes and healthcare infrastructures across the Americas, EMEA, and Asia-Pacific.
Looking forward, strategic collaborations, user-centric development, and robust regulatory planning will be critical for organizations aiming to secure leadership positions. By synthesizing these findings, decision-makers can chart a course toward sustainable innovation, optimized operations, and enhanced patient outcomes in the dynamic field of skin diagnosis.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Non Invasive Devices
- Imaging
- Dermoscopy
- Ultrasound Imaging
- Spectroscopy
- Thermal Analysis
- Imaging
- Software Solutions
- AI Platforms
- Diagnostic Apps
- Wearable Sensors
- Patches
- Wristbands
- Non Invasive Devices
- Skin Type
- Combination
- Dry
- Oily
- Application
- Acne Diagnosis
- Lesion Analysis
- Pigmentation Analysis
- Psoriasis Detection
- UV Damage Assessment
- End User
- Dermatology Centers
- Home Use
- Hospitals & Clinics
- Distribution Channel
- Direct Sales
- Distributors
- Online Sales
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Cutera, Inc.
- AGFA-Gevaert Group
- Bio-Therapeutic, Inc.
- FotoFinder Systems GmbH
- First Derm by iDoc24 Inc.
- DermTech, Inc.
- DermaSensor, Inc.
- DAVI & CIA S.p.A.
- Cynosure, Inc.
- Cortex Technology, Inc.
- Canon Medical Systems Corporation
- Canfield Scientific, Inc.
- Bomtech Electronics Co., Ltd.
- MetaOptima Technology Inc.
- Lancer Skincare, Inc.
- Koninklijke Philips N.V.
- Guangzhou Beautylife Electronic Technology Co., Ltd.
- GE Healthcare, Inc.
- Strata Skin Sciences, Inc.
- SkinVision B.V.
- Skin Analytics Ltd.
- Pixience, S.L.
- Michelson Diagnostics Ltd.
- SmartSkin Solutions, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Skin Diagnosis System market report include:- Cutera, Inc.
- AGFA-Gevaert Group
- Bio-Therapeutic, Inc.
- FotoFinder Systems GmbH
- First Derm by iDoc24 Inc.
- DermTech, Inc.
- DermaSensor, Inc.
- DAVI & CIA S.p.A.
- Cynosure, Inc.
- Cortex Technology, Inc.
- Canon Medical Systems Corporation
- Canfield Scientific, Inc.
- Bomtech Electronics Co., Ltd.
- MetaOptima Technology Inc.
- Lancer Skincare, Inc.
- Koninklijke Philips N.V.
- Guangzhou Beautylife Electronic Technology Co., Ltd.
- GE Healthcare, Inc.
- Strata Skin Sciences, Inc.
- SkinVision B.V.
- Skin Analytics Ltd.
- Pixience, S.L.
- Michelson Diagnostics Ltd.
- SmartSkin Solutions, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | November 2025 |
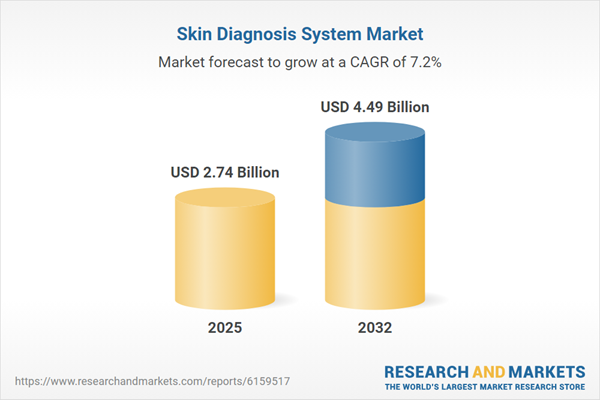
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 2.74 Billion |
| Forecasted Market Value ( USD | $ 4.49 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |