Speak directly to the analyst to clarify any post sales queries you may have.
Unveiling the Vital Role of Immune Repertoire Sequencing in Advancing Precision Medicine and Accelerating Therapeutic Development Globally
Immune repertoire sequencing has emerged as a cornerstone technology that enables unprecedented characterization of B cell and T cell receptor diversity at single-cell resolution. This approach provides a comprehensive snapshot of adaptive immune responses, revealing the molecular underpinnings of pathogen recognition, vaccine efficacy, and immunological memory. By mapping clonal expansions and tracking repertoire changes over time, researchers gain critical insights into disease progression and therapeutic mechanisms that were previously inaccessible through conventional sequencing methods.The advent of high-throughput platforms has democratized access to complex immune profiling, bridging the gap between fundamental immunology research and clinical application. As a result, translational initiatives in oncology immunotherapy, infectious disease monitoring, and autoimmune disorder management have benefited from more precise biomarker identification and patient stratification strategies. Moreover, the integration of advanced bioinformatics pipelines has streamlined data interpretation, empowering scientists and clinicians to derive actionable findings from large and multidimensional datasets.
This executive summary distills the essential trends, structural shifts, and regulatory influences shaping the immune repertoire sequencing landscape. By synthesizing segmentation insights, regional dynamics, and strategic imperatives, the analysis equips decision-makers with a cohesive understanding of the current environment and a forward-looking perspective on emerging opportunities. The following sections delve into transformative shifts, tariff impacts, market segmentation nuances, regional outlooks, competitive positioning, and tailored recommendations to guide strategic planning and investment in this rapidly evolving field.
Examining the Paradigm Shifts Driving Immune Repertoire Sequencing Technology and Its Transformative Influence on Research and Clinical Practices
Over the past decade, the immune repertoire sequencing arena has undergone a series of paradigm shifts driven by technological breakthroughs and evolving research priorities. Initially characterized by laborious protocols and limited throughputs, sequencing workflows have been revolutionized by the introduction of microfluidics-based single-cell encapsulation and droplet barcoding techniques. These innovations have accelerated sample processing and enhanced detection sensitivity, enabling researchers to profile tens of thousands of individual lymphocytes in a single run.In parallel, the emergence of cloud-based bioinformatics platforms and machine learning algorithms has transformed raw sequencing reads into biologically meaningful insights. Analysts now leverage deep learning frameworks to predict antigen receptor binding affinities and to identify public clonotypes shared among patient cohorts. These advances have catalyzed the development of more effective immunotherapeutics, from monoclonal antibodies to personalized T cell therapies.
Furthermore, collaborative consortia and open data initiatives have fostered a culture of data sharing and standardization across academic and commercial entities. As a result, reference databases for immune receptor sequences have expanded dramatically, facilitating comparative studies and meta-analyses that inform vaccine design and immunomonitoring strategies. Together, these transformative shifts have redefined the capabilities of immune repertoire sequencing, propelling the field toward more integrated and clinically impactful applications.
Analyzing the Cascading Effects of United States Tariff Measures on Reagent Supply Chains Manufacturing Costs and Collaborative Research Ecosystems
Recent tariff measures implemented by the United States have introduced a complex set of constraints on the import and pricing of critical reagents, sequencing instruments, and related components used in immune repertoire profiling. Increased duties on polymerase chain reaction consumables and library preparation reagents have elevated the cost base for laboratories, compelling many organizations to reassess supplier agreements and procurement strategies. The resultant pricing pressures have been particularly acute for multiplex PCR reagents and high-fidelity enzymes, which underpin the accuracy and throughput of clonotype detection assays.Simultaneously, tariffs on advanced sequencing platforms, including both high-capacity sequencers and compact benchtop units, have affected capital expenditure decisions for research institutes and biopharmaceutical developers. These import duties have led to extended lead times for equipment orders and a heightened emphasis on local manufacturing partnerships. In response, some instrument vendors have accelerated investments in domestic production facilities or negotiated countervailing duty exemptions through strategic alliances.
Moreover, the cumulative effect of higher input costs and logistical uncertainties has reinforced the appeal of integrated workflow services and cloud-based data analysis solutions. By outsourcing certain laboratory operations or leveraging data-centric business models, organizations can mitigate tariff-induced cost escalations. As a result, the industry is witnessing a gradual shift toward collaborative service frameworks that balance cost optimization with sustained innovation in immune repertoire sequencing.
Dissecting Key Market Segmentation Insights Highlighting Product Offerings Technology Platforms Sample Types Applications and End User Dynamics
Catering to diverse market demands, the immune repertoire sequencing ecosystem spans a comprehensive suite of offerings, from core instrumentation to specialized reagents and integrated software and services. Within the instrumentation category, research laboratories and clinical facilities rely on robust PCR machines for target amplification, as well as high-throughput sequencers to capture the full diversity of B cell and T cell repertoires. Complementing these platforms, the reagents and consumables segment comprises essential library preparation kits designed to streamline sample barcoding, multiplex PCR reagents that enable simultaneous amplification of multiple targets, and sequencing reagents optimized for accuracy and sensitivity. Underpinning this ecosystem are sophisticated data analysis software solutions, which translate raw sequence reads into actionable insights, and dedicated workflow services that offer end-to-end support from sample processing to result interpretation.From a technological standpoint, next-generation sequencing has emerged as the predominant approach, offering deep coverage and parallel processing of millions of reads, while legacy Sanger sequencing maintains relevance for targeted validation assays and smaller-scale studies that demand high sequence fidelity. The choice between these platforms often hinges on project scope, cost considerations, and required throughput levels.
Diverse sample types further enrich the market landscape, with bone marrow specimens providing direct access to central lymphoid populations, peripheral blood samples offering a minimally invasive window into circulating clonotypes, and tissue-derived biopsies enabling localized immune profiling within tumor microenvironments. Each sample category presents unique processing challenges and analytical considerations, driving demand for tailored protocols and specialized reagents.
Applications of immune repertoire sequencing span a spectrum of research and clinical use cases, including antibody discovery efforts that leverage repertoire diversity to isolate high-affinity candidates, biomarker discovery programs that correlate clonal expansions with disease states, immunodeficiency identification assays that detect repertoire collapse, and vaccine development initiatives that monitor adaptive responses to immunogens. These applications align with the needs of a broad array of end users, such as contract research organizations offering outsourced services, hospitals integrating immune monitoring into patient care pathways, pharmaceutical and biotechnology companies pursuing novel immunotherapies, and academic research institutes advancing fundamental immunology studies. Together, these segmentation insights illuminate the multifaceted nature of the immune repertoire sequencing market and underscore the interdependence of technological, application-driven, and end user dynamics.
Unraveling Regional Dynamics and Growth Drivers Shaping Immune Repertoire Sequencing Adoption Patterns Across the Americas EMEA and Asia-Pacific
In the Americas region, established research hubs and a robust biopharmaceutical sector have driven widespread adoption of immune repertoire sequencing technologies. North American institutions benefit from long-standing collaborations between academic centers and industry partners, fostering an environment conducive to clinical trials and translational research. The presence of seasoned contract research organizations and advanced sequencing service providers supports both large-scale cohort studies and niche immunology investigations. Meanwhile, Latin American markets are gradually expanding their research capabilities, spurred by government investments in life sciences infrastructure and growing demand for immunodiagnostic applications.Across Europe, the Middle East, and Africa, diverse regulatory landscapes and varying levels of research maturity create a heterogeneous profile of adoption patterns. Western European nations lead the region with significant funding earmarked for precision medicine initiatives, enabling integration of immune repertoire sequencing into oncology and autoimmune disorder research. In parallel, collaborative consortia such as pan-EMEA immunology networks promote harmonization of data standards and facilitate multicenter studies. Conversely, emerging markets in the Middle East and parts of Africa are in the early stages of capacity building, focusing on skills development and establishing foundational laboratory services. Here, partnerships with global technology providers play a pivotal role in technology transfer and workforce training.
The Asia-Pacific region exhibits remarkable growth momentum, driven by substantial investments in genomics and biotechnology across countries such as China, Japan, South Korea, and Australia. Large-scale national sequencing programs and regional precision health initiatives have accelerated adoption of high-throughput repertoire profiling. Moreover, expanding infrastructure for sample logistics and data management has enabled local laboratories to participate in global research collaborations, while increasing in-house capabilities at contract research organizations. The diversity of patient populations and disease burdens in Asia-Pacific further adds value to repository-driven studies and immunotherapeutic discovery programs. Collectively, these regional dynamics highlight the importance of tailored market strategies that address regulatory nuance, infrastructure maturity, and collaborative ecosystems across the Americas, EMEA, and Asia-Pacific.
Illuminating Strategic Postures of Leading Industry Players Spotlighting Collaborative Partnerships Innovation Investments and Competitive Differentiation Approaches
Leading providers in the immune repertoire sequencing space have adopted distinctive strategies to maintain competitive advantage. A subset of companies has prioritized vertical integration, offering seamless platforms that combine sample preparation instruments, proprietary reagent formulations, and analytics software under a unified workflow umbrella. This approach simplifies adoption for end users seeking turnkey solutions and fosters recurring revenue streams through consumable sales.Alternatively, several firms have focused on strategic alliances and co-development agreements with biopharmaceutical entities to accelerate the translation of novel immunotherapies. By aligning research efforts and sharing access to early-stage clinical data, these collaborations facilitate more rapid optimization of assay protocols and expedite candidate selection processes. Furthermore, technology vendors are increasingly partnering with specialized bioinformatics companies to enhance data interpretation capabilities, integrating machine learning modules that automate clonotype classification and epitope prediction.
In parallel, established sequencing instrument manufacturers have invested in regional service centers and training initiatives to bolster customer support infrastructure. These efforts not only reduce implementation barriers but also cultivate customer loyalty and cross-selling opportunities for newly launched products. Collectively, these strategic postures underscore the multifaceted approaches companies are employing to differentiate their offerings, expand their footprint, and respond to evolving market demands in immune repertoire sequencing.
Formulating Actionable Industry Recommendations to Enhance Supply Chain Resilience Drive Technological Innovation and Foster Cross-Sector Collaborations
In light of emerging challenges and opportunities, industry leaders should fortify supply chain resilience by diversifying procurement channels and establishing regional manufacturing partnerships. Proactive engagement with equipment and reagent suppliers can mitigate tariff-related disruptions and ensure continuity in laboratory operations. Moreover, investment in modular workflow services, including flexible on-demand sequencing and cloud-based analytics, can create adaptable platforms that accommodate shifting regulatory landscapes and customer preferences.Leaders are advised to accelerate integration of artificial intelligence and machine learning tools within data analysis pipelines to uncover deeper biological insights and drive predictive modeling. Collaborative initiatives with academic and clinical research centers can facilitate access to annotated datasets for algorithm training, enhancing the accuracy of immune repertoire interpretations. Additionally, forming industry consortiums focused on standardization of reporting formats and quality metrics will streamline cross-study comparisons and foster sector-wide benchmarks.
Finally, forging partnerships across the value chain - from sample collection to data management - will enable end-to-end solutions that reduce time to insight and optimize resource allocation. By aligning strategic investments in technology, talent development, and ecosystem collaboration, organizations can position themselves at the forefront of immune repertoire sequencing innovation and deliver tangible benefits to research and clinical communities.
Detailing Comprehensive Research Methodology Employed to Evaluate Data Sources Analytical Frameworks and Validation Processes Underpinning Market Insights
The insights presented in this executive summary are the result of a rigorous research methodology that incorporated both primary and secondary data collection. Interviews with key opinion leaders in immunology, biotechnology, and clinical laboratories provided firsthand perspectives on workflow challenges, technology adoption drivers, and application-specific requirements. These qualitative inputs were supplemented by an exhaustive review of peer-reviewed literature, patent filings, regulatory guidance documents, and corporate disclosures to ensure comprehensive coverage of technological trends and strategic initiatives.Analytical frameworks were applied to categorize market segmentation dimensions, delineating offering types, technological platforms, sample sources, application areas, and end user segments. Regional analyses were conducted via a bottom-up approach, examining infrastructure maturity, funding patterns, and collaborative networks across major geographies. Validation exercises involved cross-referencing data points with reported case studies, conference proceedings, and industry consortium outputs to confirm consistency and reliability.
The combination of expert interviews, secondary research, and validation protocols has yielded a robust dataset that underpins the findings and recommendations of this report. This structured approach ensures that the conclusions reflect both current market realities and emerging opportunities in the immune repertoire sequencing landscape.
Concluding Strategic Reflections on Emerging Opportunities Challenges and Collaborative Pathways to Propel Immune Repertoire Sequencing Advancements Forward
The immune repertoire sequencing field stands at a pivotal juncture, shaped by technological innovation, evolving research paradigms, and global policy dynamics. The convergence of high-throughput platforms, sophisticated bioinformatics, and collaborative ecosystems has expanded the scope of applications from fundamental immunology to targeted therapeutic development. Regulatory influences and tariff-driven constraints continue to recalibrate cost structures and supply chain strategies, underscoring the need for agile operational models.Segmentation insights reveal a diverse landscape of offerings and use cases, while regional analyses highlight distinct adoption patterns and infrastructure readiness across the Americas, EMEA, and Asia-Pacific. Leading companies are leveraging vertical integration, strategic partnerships, and service-oriented models to differentiate their offerings and capture emerging opportunities. Actionable recommendations emphasize supply chain diversification, AI integration, and consortium-driven standardization as key levers for sustained growth and innovation.
In sum, stakeholders who embrace collaborative frameworks, invest in advanced analytics, and maintain operational flexibility will be best positioned to drive the next wave of discoveries and deliver impactful outcomes in immune repertoire sequencing.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Offerings
- Instruments
- PCR Machines
- Sequencers
- Reagents & Consumables
- Library Preparation Kits
- Multiplex PCR Reagents
- Sequencing Reagents
- Software & Services
- Data Analysis Software
- Workflow Services
- Instruments
- Technology
- Next Generation Sequencing
- Sanger Sequencing
- Sample Type
- Bone Marrow
- Peripheral Blood
- Tissue Sample
- Application
- Antibody Discovery
- Biomarker Discovery
- Immunodeficiency Identification
- Vaccine Development
- End User
- Contract Research Organization
- Hospital
- Pharmaceutical & Biotech Companies
- Research Institute
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Adaptive Biotechnologies Corporation
- BGI Genomics Co., Ltd.
- Thermo Fisher Scientific Inc.
- Illumina, Inc.
- 10x Genomics, Inc.
- QIAGEN N.V.
- Pacific Biosciences of California, Inc.
- Takara Bio Inc.
- ArcherDX, Inc.
- Agilent Technologies, Inc.
- CD Genomics
- Creative Biolabs, Inc.
- Eurofins Genomics
- F. Hoffmann-La Roche Ltd.
- GENEWIZ by Azenta Life Sciences
- Invitae Corp.
- iRepertoire, Inc.
- MedGenome Inc.
- NanoString Technologies, Inc. by Bruker Corporation
- Paragon Genomics, Inc.
- Twist Bioscience Corporation
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Immune Repertoire Sequencing market report include:- Adaptive Biotechnologies Corporation
- BGI Genomics Co., Ltd.
- Thermo Fisher Scientific Inc.
- Illumina, Inc.
- 10x Genomics, Inc.
- QIAGEN N.V.
- Pacific Biosciences of California, Inc.
- Takara Bio Inc.
- ArcherDX, Inc.
- Agilent Technologies, Inc.
- CD Genomics
- Creative Biolabs, Inc.
- Eurofins Genomics
- F. Hoffmann-La Roche Ltd.
- GENEWIZ by Azenta Life Sciences
- Invitae Corp.
- iRepertoire, Inc.
- MedGenome Inc.
- NanoString Technologies, Inc. by Bruker Corporation
- Paragon Genomics, Inc.
- Twist Bioscience Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
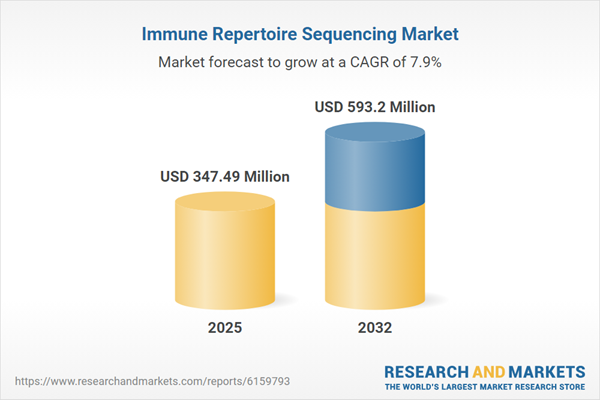
| Estimated Market Value ( USD | $ 347.49 Million |
| Forecasted Market Value ( USD | $ 593.2 Million |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |