Speak directly to the analyst to clarify any post sales queries you may have.
Setting the Stage for Hormonal Transdermal Patch Innovations and Market Evolution in Women's Health Solutions Worldwide
Hormonal transdermal patches have emerged as a cornerstone in women's health care, combining pharmacological precision with patient convenience through controlled, non-invasive delivery of key hormones. By bypassing gastrointestinal pathways, these delivery systems optimize bioavailability while minimizing systemic peaks and troughs, thereby improving therapeutic outcomes and user adherence. Rapid advancements in adhesive matrices and rate-controlled reservoir technologies have expanded formulation possibilities, allowing a wider spectrum of hormone combinations and dose profiles.Against this backdrop of innovation, executive stakeholders require a cohesive synthesis of market drivers, regulatory currents, and technological breakthroughs. This executive summary distills critical insights into the evolving hormonal transdermal patch ecosystem, offering strategic guidance on emerging opportunities, risk factors, and best practices. It serves as a navigational tool for decision-makers aiming to align product pipelines, operational strategies, and commercial approaches with the latest industry trends.
Unveiling the Key Technological and Regulatory Shifts Driving the Hormonal Transdermal Patch Ecosystem to New Heights
The landscape of hormonal transdermal patch development is undergoing profound transformation, driven by breakthroughs in polymer science, microscale drug distribution, and digital health integration. New adhesive formulations enable customized wear times, mitigating skin irritation and enhancing patient comfort. Simultaneously, miniaturized reservoir designs offer fine-tuned hormone release, accommodating personalized dosing regimens for contraception or menopausal symptom management.Regulatory bodies are responding with updated guidance on risk-benefit evaluation, emphasizing post-market surveillance and real-world evidence to ensure long-term safety. In parallel, patient expectations have shifted toward discreet, user-friendly formats, catalyzing cross-sector collaborations among pharmaceutical developers, medical device engineers, and software innovators. Consequently, the hormonal patch arena is reconciling stringent quality standards with agile, consumer-centered design philosophies.
Assessing the Far-Reaching Effects of United States Tariff Adjustments on Hormonal Transdermal Patch Supply Chains and Cost Structures
The implementation of fresh tariffs on imported polymers, adhesive compounds, and certain packaging inputs has reshaped the cost structure of hormonal transdermal patch manufacturing. Increased duties have compelled manufacturers to reassess supply chain configurations, weighing the trade-offs between global procurement and domestic sourcing. Some organizations have pursued nearshoring strategies to secure critical raw materials and reduce lead times, while others have renegotiated long-term contracts to spread tariff-related expenses evenly across production cycles.These trade policy shifts extend beyond input costs, influencing strategic alliances and contract manufacturing relationships. Manufacturers are forging deeper partnerships with specialized suppliers to co-develop tariff-resilient materials and optimize batch production processes. At the same time, scenario planning and risk modeling have become integral to corporate planning sessions, ensuring that price adjustments align with patient affordability and payer reimbursement frameworks without compromising quality or compliance.
Delineating Critical Segmentation Insights to Illuminate Diverse Therapeutic, Technological, and End User Trends in Patch Applications
In examining the hormonal transdermal patch sector through a product lens, combined hormonal patches occupy a central role in addressing both contraceptive and menopausal management needs, whereas estrogen-only formulations cater to targeted vasomotor symptom relief. Progestin-only products have gained traction among users seeking hormone-specific therapies. Parallel advances in patch technology distinguish matrix systems-where active ingredients are uniformly incorporated into the adhesive substrate-from reservoir designs that leverage discrete drug chambers for sustained release and dose flexibility.Therapeutic segmentation further clarifies market dynamics: contraceptive applications demand precise release profiles and cycle management, while menopause management patches prioritize steady hormone delivery to alleviate long-term quality-of-life symptoms. End-user considerations span self-administered home healthcare environments, where ease of use and patient education are paramount, to hospitals and clinics that require batch-level quality assurance, traceability, and integration with broader treatment protocols.
Mapping Regional Dynamics and Growth Drivers Across the Americas, EMEA, and Asia-Pacific Hormonal Transdermal Patch Markets
Regional dynamics reveal distinctly nuanced growth trajectories and strategic imperatives across the Americas, EMEA, and Asia-Pacific markets. In the Americas, established regulatory pathways and well-defined reimbursement mechanisms have facilitated rapid adoption of next-generation patch formats. Healthcare providers and insurers are increasingly receptive to products that combine robust clinical profiles with cost-effective delivery methods, fueling collaborative pilot programs and real-world studies.The EMEA region presents a mosaic of market access challenges, with cost containment policies, extended approval cycles, and generics competition shaping pricing strategies. Yet, strong patient advocacy networks and innovative funding models in select European markets have created early opportunities for differentiated transdermal therapies. Meanwhile, Asia-Pacific is experiencing surging demand driven by expanding middle-class populations, greater healthcare spending, and emerging pharmaceutical manufacturing hubs, positioning the region as both a key consumer and production center for hormonal patch offerings.
Highlighting Major Industry Players' Strategic Moves and Competitive Positioning in the Hormonal Transdermal Patch Space
The competitive landscape features a blend of established multinational pharmaceutical companies, specialty biotech firms, and agile start-ups, each contributing distinct capabilities to the hormonal transdermal patch ecosystem. Large-scale drug developers leverage extensive clinical trial infrastructures and global distribution channels to introduce incremental innovations, whereas mid-tier players often focus on niche formulations, rapid regulatory filings, and strategic licensing agreements to gain market footholds.Emerging entrants are forging cross-disciplinary alliances, uniting expertise in polymer engineering, bioadhesive technologies, and digital health solutions to enhance patient engagement and therapeutic adherence. Strategic mergers, acquisitions, and contract manufacturing partnerships underscore a trend toward consolidation and vertical integration, as firms seek to streamline R&D workflows and secure end-to-end supply chain visibility.
Strategic Recommendations for Industry Stakeholders to Optimize Innovation, Partnerships, and Market Penetration in Hormonal Transdermal Delivery
Industry leaders are encouraged to prioritize investment in advanced drug delivery platforms that integrate sensor-enabled adhesives and companion digital health applications. By collaborating early with regulatory authorities and key opinion leaders, organizations can de-risk development timelines and align product differentiation with evolving safety guidelines. Developing robust intellectual property strategies around novel compounds and device configurations will safeguard competitive advantage in a fragmented patent landscape.Moreover, establishing resilient supply chains through dual sourcing and supplier co-development agreements can mitigate trade-related disruptions. Tailoring commercialization tactics to regional healthcare frameworks-such as value-based contracting in North America or collaborative research initiatives in Europe-will enhance market entry effectiveness. Finally, ongoing engagement with patient communities to co-create educational resources will drive adherence and brand loyalty.
Explaining a Comprehensive, Multi-Method Research Framework Underpinning Insights into Hormonal Transdermal Patch Trends and Drivers
This analysis synthesizes insights derived from a mixed-method research approach combining primary qualitative interviews with endocrinologists, patch developers, regulatory specialists, and procurement executives, alongside secondary review of scientific literature, policy documents, and technology briefs. Data triangulation ensures consistency and completeness, with thematic coding applied to identify core drivers, challenges, and emerging trends within the hormonal patch domain.Complementing qualitative findings, competitive benchmarking provides context on product pipelines, approval timelines, and strategic alliances. Each hypothesis was validated through multiple stakeholder consultations, and regional analyses were calibrated against publicly available policy frameworks and industry press releases. Rigorous peer review by subject matter experts ensures that all conclusions reflect the most current, evidence-based perspectives.
Synthesizing Core Findings to Illuminate Future Trajectories and Opportunities in Hormonal Transdermal Patch Innovation and Market Expansion
Bringing together technological breakthroughs, evolving regulatory requirements, and shifting patient preferences, the hormonal transdermal patch market stands at a pivotal juncture. Diverse segmentation pathways-from combined and hormone-specific formulations to matrix and reservoir platforms-highlight the industry's capacity for tailored therapy. Regional variations underscore the importance of adaptive market entry strategies, while tariff-induced supply chain adjustments emphasize the need for operational resilience.Competitive intensity continues to drive collaboration across pharmaceutical, device, and digital health sectors, fostering an ecosystem where agility and strategic foresight define success. By integrating the recommendations outlined herein, stakeholders can harness these insights to craft robust product roadmaps, optimize resource allocation, and ultimately enhance patient outcomes in women's health.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Combined Hormonal Patches
- Estrogen-only Patches
- Progestin-only Patches
- Patch Technology
- Matrix Patch
- Reservoir Patch
- Therapeutic Area
- Contraception
- Menopause Management
- End User
- Home Healthcare
- Hospitals & Clinics
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- AbbVie Inc.
- Allergan plc
- AdhexPharma, SAS
- Amneal Pharmaceuticals LLC.
- Bayer AG
- Bliss GVS Pharma Limited
- Johnson & Johnson Services, Inc.
- LTS Lohmann Therapie Systeme AG
- Luye Pharma Group
- Mylan N.V.
- Novartis AG
- Noven Pharmaceuticals, Inc.
- Sandoz International GmbH
- Teva Pharmaceutical Industries Ltd
- Viatris Inc.
- Zydus Lifesciences Ltd
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Hormonal Transdermal Patches market report include:- AbbVie Inc.
- Allergan plc
- AdhexPharma, SAS
- Amneal Pharmaceuticals LLC.
- Bayer AG
- Bliss GVS Pharma Limited
- Johnson & Johnson Services, Inc.
- LTS Lohmann Therapie Systeme AG
- Luye Pharma Group
- Mylan N.V.
- Novartis AG
- Noven Pharmaceuticals, Inc.
- Sandoz International GmbH
- Teva Pharmaceutical Industries Ltd
- Viatris Inc.
- Zydus Lifesciences Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
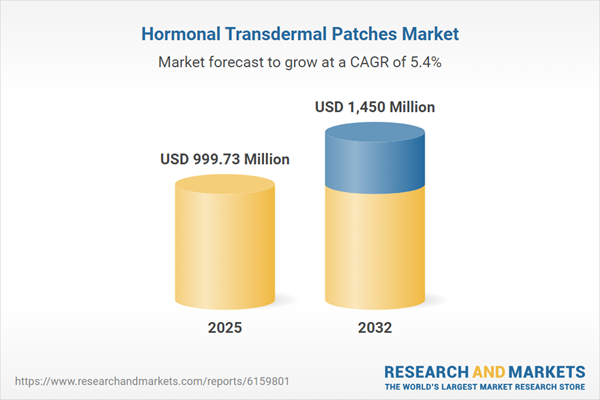
| Estimated Market Value ( USD | $ 999.73 Million |
| Forecasted Market Value ( USD | $ 1450 Million |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 17 |