Speak directly to the analyst to clarify any post sales queries you may have.
A clear foundational overview that contextualizes residual DNA testing concepts, laboratory requirements, regulatory touchpoints, and stakeholder priorities for strategic decision-making
Residual DNA testing stands at the intersection of advancing molecular biology techniques and expanding practical applications in medicine, forensics, and research. This introduction establishes the foundational concepts necessary to understand residual DNA testing as a discipline: the types of residual DNA encountered in clinical and investigative settings, the analytical constraints laboratory teams face, and the operational workflows that influence data quality and turnaround time. The intent of this section is to orient technical leaders, procurement officers, and executive decision-makers to the core capabilities and limitations of contemporary residual DNA workflows, while also highlighting areas where investment and process redesign can yield meaningful gains in sensitivity and interpretability.To set expectations, the narrative frames residual DNA testing within the broader regulatory and quality assurance environment that governs molecular diagnostics and forensic evidence handling. It emphasizes standardization needs, traceability of sample provenance, and the importance of validated reagents and instruments for reproducible outcomes. Finally, the introduction outlines the report's structure and analytical approach so readers can quickly locate sections most relevant to their role, whether that is technology selection, service contracting, or research collaboration.
How advances in sequencing chemistry, automation, and analytics are reshaping residual DNA testing and redefining operational and regulatory expectations across the ecosystem
The landscape of residual DNA testing is undergoing transformative shifts driven by rapid advances in sequencing chemistry, automation, and data analytics. Over recent years, next-generation sequencing platforms have improved per-run throughput and sensitivity, enabling laboratories to detect lower-template and degraded DNA with greater confidence. Concurrently, improvements in library preparation and targeted enrichment strategies have expanded the practical applications of residual DNA testing beyond classical short tandem repeat profiling to include targeted single-nucleotide variant detection and metagenomic screening in degraded samples.In parallel, automation and integrated workflows have reduced manual handling and contamination risks, while cloud-enabled bioinformatics and machine learning tools have accelerated variant interpretation and pattern recognition in complex mixtures. These changes are complemented by evolving regulatory expectations that emphasize validation, harmonized reporting standards, and chain-of-custody controls for forensic specimens and clinical leftovers. Stakeholders should also note growing interest in decentralized testing models and partnerships that combine centralized sequencing capacity with local sample intake, which together are reshaping service delivery and competitive dynamics across the ecosystem.
An assessment of how recent United States tariff measures in 2025 have altered supply chain resilience, procurement strategies, and operational planning for residual DNA testing stakeholders
The cumulative effect of recent United States tariff actions and trade policy changes in 2025 has incrementally affected the supply chains that support residual DNA testing workflows. Tariff-induced increases in the landed cost of imported instruments, reagents, and consumables have created pressure on procurement budgets and prompted organizations to reassess supplier diversity and sourcing strategies. In response, many laboratories and manufacturers have prioritized near-shore sourcing, negotiated longer-term vendor agreements to stabilize pricing, and accelerated qualification of alternative suppliers to mitigate exposure to single-origin dependencies.Beyond direct cost implications, tariffs have influenced strategic decisions around inventory management and capital spending. Laboratory leaders have shifted toward just-in-case inventory buffers for critical reagents while also evaluating lease and service models for high-value instruments to reduce upfront capital outlays. For manufacturers, tariffs have reinforced incentives to localize certain production stages and to cultivate domestic supply partners for key electronic and reagent components. Together, these adjustments are shaping a more resilient but operationally complex ecosystem in which trade policy considerations are an integral part of procurement and deployment planning.
Deep segmentation-driven insights that align instruments, reagents, technologies, sample types, applications, and end-user needs to inform product strategy and service positioning
Segment-level insights reveal how residual DNA testing solutions map to distinct product, technology, sample type, application, and end-user requirements, and how these axes interact to shape purchasing and operational behavior. When viewed by product, the market comprises instruments, kits and reagents, and services. Instruments include electrophoresis systems, PCR instruments, and sequencers, each serving different throughput and sensitivity needs. Kits and reagents span extraction kits, NGS reagents, and PCR kits, and their selection strongly influences downstream data quality and assay robustness. Services cover custom solutions and outsourcing, enabling laboratories to augment internal capabilities or access specialized workflows on demand.Viewed through the technology lens, capillary electrophoresis remains a workhorse for fragment analysis while microarray analysis, including CGH array and SNP array formats, addresses certain genomic interrogation needs. Next generation sequencing approaches, differentiated by targeted sequencing and whole genome sequencing, provide complementary sensitivity and breadth, whereas polymerase chain reaction modalities-both digital and quantitative-offer rapid, focused detection when throughput and speed are priorities. Sample-type segmentation highlights operational variability: blood specimens, whether whole blood or dried blood spots, present distinct extraction and stability considerations; saliva offers noninvasive collection advantages but requires careful inhibition control; tissue analysis, from fresh to frozen to paraffin embedded formats, demands tailored extraction and pre-analytic handling.
Application-based segmentation differentiates clinical diagnostics, forensic testing, and research applications. Clinical diagnostics covers genetic disorder screening and oncology applications with tight regulatory and clinical validation demands, while forensic testing emphasizes evidentiary chain-of-custody and mixture interpretation. Research applications span academic research and pharma R&D, where flexibility and exploratory assay design are prioritized. Finally, end-user segmentation identifies diagnostic centers, hospitals, and research institutions; research institutions further divide into academic institutions and biotech and pharma companies, each with unique purchasing cycles, compliance expectations, and in-house capabilities. Together, these segmentation perspectives enable more precise alignment of product development, service offerings, and go-to-market strategies.
Regional variations in adoption, regulation, and commercialization that determine how residual DNA testing technologies are deployed and supported across global markets
Regional dynamics materially influence how residual DNA testing technologies are adopted, regulated, and commercialized across different markets. In the Americas, investment in centralized sequencing infrastructure and forensic modernization programs supports broad adoption, while private laboratories and hospital networks drive adoption in clinical diagnostics through strategic partnerships and service contracts. Transitioning to Europe, Middle East & Africa, the regulatory landscape is heterogeneous: certain jurisdictions emphasize harmonized standards and robust accreditation frameworks, whereas others are focused on capacity building and technology transfer programs, creating varied demand profiles for instruments and services.Asia-Pacific exhibits a mixed profile of rapid technology uptake in urban centers, increasing domestic manufacturing capacity for reagents and instruments, and an expanding base of academic and industry research that fuels demand for specialized services. Cross-region differences in reimbursement practices, regulatory timelines, and public-sector investment priorities are significant factors that shape commercialization strategies. Moreover, logistics complexity, import/export regulatory requirements, and localization preferences all influence how companies and laboratories sequence market entry, supply agreements, and training programs across these regional clusters.
Strategic company behaviors highlighting platform innovation, partnerships, and service differentiation that are shaping competitive advantage and ecosystem integration
Key company dynamics reflect a strategic blend of product innovation, service expansion, and collaborative partnerships. Leading instrument manufacturers continue to invest in platform modularity, interoperability, and automation to reduce hands-on time and contamination risk, while reagent and kit providers are prioritizing robustness and room-temperature stability to ease logistics. Service providers are differentiating through rapid turnaround, chain-of-custody rigor, and bespoke bioinformatics pipelines that streamline interpretation of low-template and mixed-source DNA.Across the ecosystem, companies are forging partnerships with academic centers and clinical laboratories to validate niche applications and to generate real-world performance evidence. Mergers and acquisitions, strategic minority investments, and licensing deals are being used to accelerate entry into adjacent capabilities, particularly in areas such as targeted sequencing panels, sample extraction automation, and forensic-grade interpretation software. Additionally, several market participants are developing training and certification programs that help end users meet accreditation requirements while reducing implementation friction. These strategic moves collectively indicate a market that is evolving through focused horizontal and vertical integration to deliver end-to-end solutions.
Five pragmatic and actionable recommendations that industry leaders can implement to strengthen operational resilience, compliance, and commercial advantage in residual DNA testing
Industry leaders must adopt pragmatic, forward-looking actions to capture value from the evolving residual DNA testing landscape. First, invest in validated workflows that emphasize pre-analytic controls and chain-of-custody documentation to ensure data integrity and regulatory compliance. This foundational work reduces downstream rework and increases confidence in interpretations that inform clinical or forensic decisions. Second, diversify supplier footprints for critical instruments and reagents to lower exposure to trade-policy volatility and to maintain operational continuity, while also evaluating consignment, lease, and managed-service alternatives to manage capital and supply risk.Third, allocate resources to automation and LIMS integration to minimize manual handling and to standardize processes across sites, which improves throughput and reduces contamination risk. Fourth, strengthen bioinformatics and interpretation capabilities through modular pipelines and well-documented validation, enabling rapid interpretation of low-template, degraded, or mixed DNA samples with reproducible reporting. Fifth, pursue collaborative validation studies and training partnerships with accredited laboratories to accelerate adoption while supporting accreditation and legal defensibility. Finally, align commercial strategies with regional regulatory expectations and logistics realities to ensure that product and service rollouts are both compliant and commercially viable. Together, these recommendations help organizations convert technological advances into operational advantage and sustained stakeholder trust.
A rigorous mixed-methods research methodology combining literature review, expert interviews, technology assessment, and quality assurance to ensure credible and actionable insights
The research methodology underpinning this analysis integrates multiple complementary approaches to ensure robustness, credibility, and practical relevance. The process began with a systematic review of peer-reviewed literature, regulatory guidance documents, technical white papers, and vendor product specifications to establish a technical baseline and to identify validated methods and standards. Primary research activities included interviews with laboratory directors, procurement leads, and technology specialists across clinical, forensic, and academic settings to capture operational realities, unmet needs, and adoption barriers.To validate qualitative insights, the study incorporated comparative technology assessments and protocol reviews, focusing on extraction efficiency, inhibitor mitigation, library preparation robustness, and interpretation pipelines across representative platforms. Data synthesis emphasized triangulation across sources, reconciliation of divergent viewpoints, and transparent documentation of assumptions. Quality assurance steps included expert peer review of methodological choices, reproducibility checks on key procedural descriptions, and clear traceability of evidence for each major claim. Ethical considerations and chain-of-custody contexts were treated as critical inputs when evaluating forensic workflows and clinical specimen use, and confidentiality protections were applied to primary interview data to preserve source anonymity.
A concise synthesis of actionable conclusions that link technology maturity, operational resilience, and strategic alignment to future leadership in residual DNA testing
Residual DNA testing is at a pivotal moment where technological maturation, supply chain realities, and regulatory expectations converge to create both challenges and opportunities. The cumulative narrative of this report highlights the importance of validated pre-analytic controls, careful selection of assays and platforms, and organizational investment in automation and interpretive capacity. At the same time, trade policy and regional differences underscore the need for procurement agility and supplier diversification to sustain operations and protect timelines.Looking forward, stakeholders that prioritize methodological rigor, operational resilience, and strategic partnerships will be best positioned to translate technological capabilities into reliable outcomes for clinical, forensic, and research use cases. The pathway to sustained value lies in aligning product and service development with accreditation requirements, region-specific deployment strategies, and robust training programs that lower implementation friction. In sum, integrating technical excellence with pragmatic commercial and operational planning will determine which organizations lead the next wave of adoption and impact in residual DNA testing.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Product
- Instruments
- Electrophoresis Systems
- PCR Instruments
- Sequencers
- Kits And Reagents
- Extraction Kits
- NGS Reagents
- PCR Kits
- Services
- Custom Solutions
- Outsourcing
- Instruments
- Technology
- Capillary Electrophoresis
- Microarray Analysis
- CGH Array
- SNP Array
- Next Generation Sequencing
- Targeted Sequencing
- Whole Genome Sequencing
- Polymerase Chain Reaction
- Digital
- Quantitative
- Sample Type
- Blood
- Dried Blood Spots
- Whole Blood
- Saliva
- Tissue
- Fresh Tissue
- Frozen Tissue
- Paraffin Embedded
- Blood
- Application
- Clinical Diagnostics
- Genetic Disorder Screening
- Oncology
- Forensic Testing
- Research Applications
- Academic Research
- Pharma R&D
- Clinical Diagnostics
- End User
- Diagnostic Centers
- Hospitals
- Research Institutions
- Academic Institutions
- Biotech And Pharma Companies
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- ATCC
- Bio‑Rad Laboratories, Inc.
- Charles River Laboratories International, Inc.
- Creative Biogene Co., Ltd.
- Cygnus Technologies Ltd.
- Eagle Biosciences, Inc.
- Eurofins Scientific (Ireland) Limited
- ExCell Bio Group
- FUJIFILM Wako Pure Chemical Corporation
- Generi Biotech S.L.
- Intertek Group plc
- Jiangsu Hillgene Biopharma Co., Ltd.
- Merck KGaA
- Minerva Biolabs GmbH
- MtoZ Biolabs Inc.
- QIAGEN N.V.
- RayKol Group Corp., Ltd.
- F. Hoffmann-La Roche AG
- Shanghai Jinbo Biotechnology Co., Ltd.
- Thermo Fisher Scientific Inc.
- Wuhan Hzymes Biotechnology Co., Ltd.
- Yeasen Biotechnology (Shanghai) Co., Ltd.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Residual DNA Testing market report include:- ATCC
- Bio‑Rad Laboratories, Inc.
- Charles River Laboratories International, Inc.
- Creative Biogene Co., Ltd.
- Cygnus Technologies Ltd.
- Eagle Biosciences, Inc.
- Eurofins Scientific (Ireland) Limited
- ExCell Bio Group
- FUJIFILM Wako Pure Chemical Corporation
- Generi Biotech S.L.
- Intertek Group plc
- Jiangsu Hillgene Biopharma Co., Ltd.
- Merck KGaA
- Minerva Biolabs GmbH
- MtoZ Biolabs Inc.
- QIAGEN N.V.
- RayKol Group Corp., Ltd.
- F. Hoffmann-La Roche AG
- Shanghai Jinbo Biotechnology Co., Ltd.
- Thermo Fisher Scientific Inc.
- Wuhan Hzymes Biotechnology Co., Ltd.
- Yeasen Biotechnology (Shanghai) Co., Ltd.
Table Information
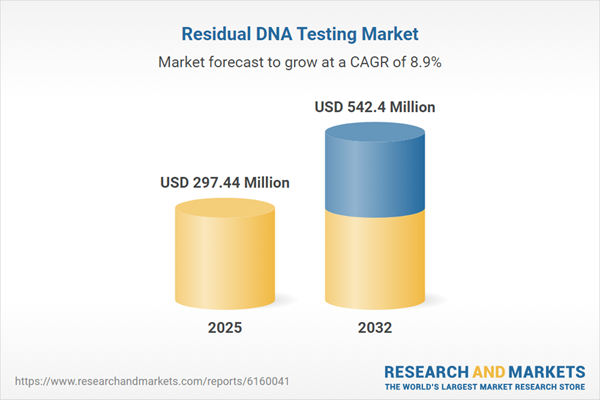
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 297.44 Million |
| Forecasted Market Value ( USD | $ 542.4 Million |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |